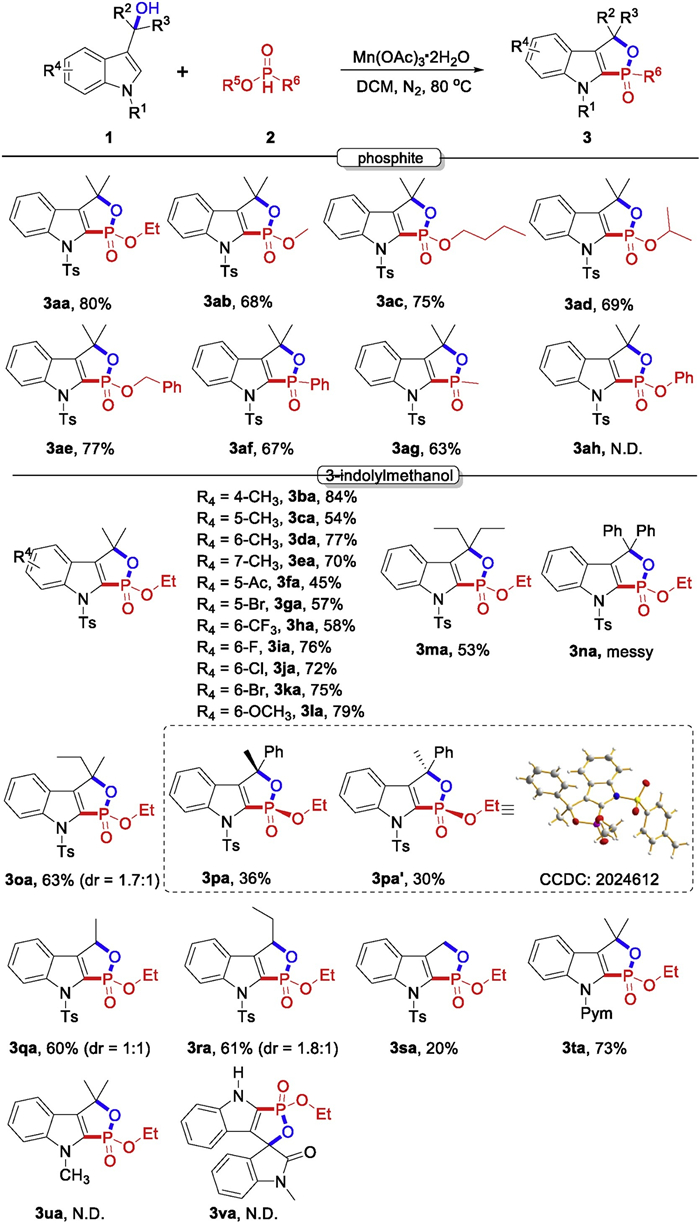
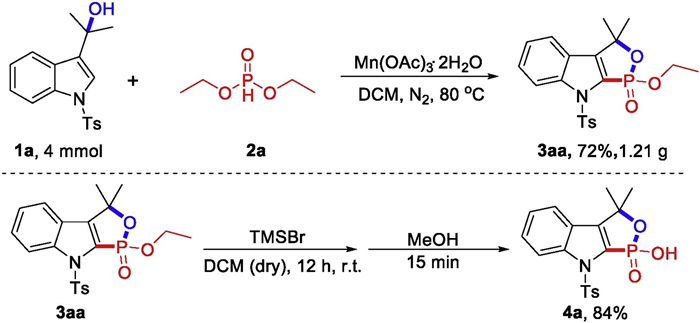
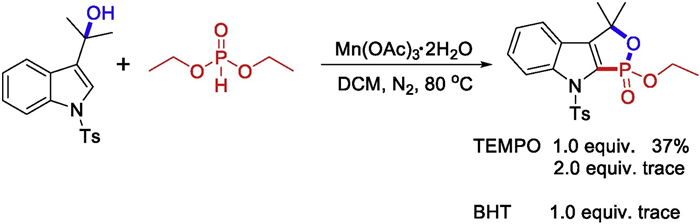
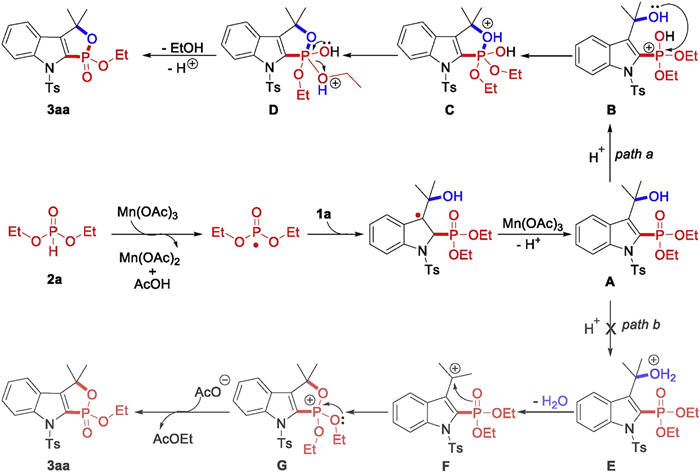
-
[1]
(a) W.J. Tang, X.M. Zhang, Chem. Rev. 103 (2003) 3029-3070;
(b) H.A. McManus, P.J. Guiry, Chem. Rev. 104 (2004) 4151-4202;
(c) S.F. Zhu, Q.L. Zhou, Acc. Chem. Res. 50 (2017) 988-1001;
(d) J.M. John, S.H. Bergens, Angew. Chem. Int. Ed. 50 (2011) 10377-10380;
(e) W.L. Yang, H. Wang, Z.R. Pan, Z. Li, W.P. Deng, Chin. Chem. Lett. 31 (2020) 721-724.
-
[2]
(a) C. Queffélec, M. Petit, P. Janvier, D.A. Knight, B. Bujoli, Chem. Rev. 112 (2012) 3777-3807;
(b) J.L. Montchamp, Acc. Chem. Res. 47 (2014) 77-87;
(c) S.O. Jeon, J.Y. Lee, J. Mater. Chem. 22 (2012) 7239-7244.
-
[3]
(a) E.D. Clercq, Med. Res. Rev. 31 (2011) 118-160;
(b) F.R. Alexandre, A. Amador, S. Bot, et al., J. Med. Chem. 54 (2011) 392-395;
(c) X.J. Zhou, R.C. Garner, S. Nicholson, C.J. Kissling, D. Mayers, J. Clin. Pharmacol. 49 (2009) 1408-1416.
-
[4]
(a) P.G. Syam, B.B. Hari, R.C. Naga, C.D. Reddy, S. Reddy, Synth. Commun. 39 (2009) 1310-1316;
(b) K. Vanya, D. Asya, K. Karamfil, E. Dobromir, Genetika 41 (2009) 179-188;
(c) V.V. Belakhov, M.A. Shneider, E.V. Komarov, B.I. Ionin, Khim. Farm. Zh. 22 (1988) 719-724.
-
[5]
(a) F. Yu, X.D. Lian, S.M. Ma, Org. Lett. 9 (2007) 1703-1706;
(b) Y. Lv, G.B. Hu, D.M. Ma, et al., J. Org. Chem. 80 (2015) 6908-6914;
(c) I. Essid, C. Laborde, F. Legros, et al., Org. Lett. 19 (2017) 1882-1885;
(d) T. Ryu, J. Kim, Y. Park, S. Kim, P.H. Lee, Org. Lett. 15 (2013) 3986-3989;
(e) D. Eom, Y. Jeong, Y.R. Kim, et al., Org. Lett. 15 (2013) 5210-5213.
-
[6]
(a) C.X. Zhuo, C. Zheng, S.L. You, Acc. Chem. Res. 47 (2014) 2558-2573;
(b) R.J. Phipps, N.P. Grimster, M.J. Gaunt, J. Am. Chem. Soc. 130 (2008) 8172-8174;
(c) S.D. Yang, C.L. Sun, Z. Fang, et al., Angew. Chem. Int. Ed. 47 (2008) 1473-1476;
(d) Z.Z. Shi, S.T. Ding, Y.X. Cui, N. Jiao, Angew. Chem. Int. Ed. 48 (2009) 7895-7898;
(e) X. Chen, K.M. Engle, D.H. Wang, J.Q. Yu, Angew. Chem. Int. Ed. 48 (2009) 5094-5115;
(f) E.F. Huang, L. Zhang, C.Y. Xiao, et al., Chin. Chem. Lett. 30 (2019) 2157-2159;
(g) T.D. Tan, X.Q. Zhu, M. Jia, et al., Chin. Chem. Lett. 31 (2020) 1309-1312.
-
[7]
(a) Y.C. Zhang, F. Jiang, F. Shi, Acc. Chem. Res. 53 (2020) 425-446;
(b) G.J. Mei, F. Shi, J. Org. Chem. 82 (2017) 7695-7707;
(c) L. Wang, Y.Y. Chen, J. Xiao, Asian J. Org. Chem. 3 (2014) 1036-1052;
(d) X.P. Han, H. Li, R.P. Hughes, J. Wu, Angew. Chem. Int. Ed. 51 (2012) 10390-10393;
(e) Y.C. Xiao, Q.Q. Zhou, L. Dong, T.Y. Liu, Y.C. Chen, Org. Lett. 14 (2012) 5940-5943;
(f) H. Wen, L. Wang, L.B. Xu, et al., Adv. Synth. Catal. 357 (2015) 4023-4030;
(g) J. Xiao, H. Wen, L. Wang, et al., Green. Chem. 18 (2016) 1032-1037;
(h) Y.Z. Zou, C.L. Chen, X.X. Chen, X.X. Zhang, W.D. Rao, Eur. J. Org. Chem. 2017 (2017) 2266-2271.
-
[8]
N.A. Kogan, G.N. Kulbitskii, Chem. Heterocycl. Compd. 14(1978) 46-48.
doi: 10.1007/BF00635941
-
[9]
(a) K.T. Potts, J. Org. Chem. 26 (1961) 4719-4721;
(b) X.F. Zeng, S.J. Ji, S.Y. Wang, Tetrahedron 61 (2005) 10235-10241;
(c) J. Huang, G.X. Li, G.B. Yang, J.Z. Zhao, Z. Tang, Asian J. Org. Chem. 6 (2017) 1741-1744;
(d) M.Y. Xiao, D.D. Ren, L.B. Xu, et al., Org. Lett. 19 (2017) 5724-5727;
(e) J.A. Mackay, R.L. Bishop, V.H. Rawal, Org. Lett. 7 (2005) 3421-3424;
(f) L.J. Zhou, Y.C. Zhang, J.J. Zhao, F. Shi, S.J. Tu, J. Org. Chem. 79 (2014) 10390-10398.
-
[10]
(a) Y.X. Zou, X.Y. Liu, J. Zhang, et al., Adv. Synth. Catal. 361 (2019) 5311-5316;
(b) X. Li, W. Tan, Y.X. Gong, F. Shi, J. Org. Chem. 80 (2015) 1841-1848;
(c) Y. Liu, R.Y. Zhu, H.Z. Li, Y. Jiang, F. Shi, Synthesis 47 (2015) 1436-1446.
-
[11]
(a) M. Rueping, B.J. Nachtsheim, S.A. Moreth, Angew. Chem. Int. Ed. 47 (2008) 593-596;
(b) C. Guo, J. Song, J.Z. Huang, et al., Angew. Chem. Int. Ed. 51 (2012) 1046-1050;
(c) C. Ma, F. Jiang, F.T. Sheng, et al., Angew. Chem. Int. Ed. 58 (2019) 3014-3020;
(d) L. Song, Q.X. Guo, X.C. Li, J. Tian, Y.G. Peng, Angew. Chem. Int. Ed. 51 (2012) 1899-1902;
(e) Y. Liu, H.H. Zhang, Y.C. Zhang, et al., Chem. Commun. 50 (2014) 12054-12057;
(f) F.L. Sun, M. Zeng, Q. Gu, S.L. You, Chem. Eur. J. 15 (2019) 8709-8712.
-
[12]
(a) B. Xu, Z.L. Guo, W.Y. Jin, et al., Angew. Chem. Int. Ed. 51 (2012) 1059-1062;
(b) W. Tan, X. Li, Y.X. Gong, M.D. Ge, F. Shi, Chem. Commun. 50 (2014) 15901-15904;
(c) F. Shi, H.H. Zhang, X.X. Sun, et al., Chem. Eur. J. 21 (2015) 3465-3471.
-
[13]
(a) P. Malik, R. Bhushan, New J. Chem. 41 (2017) 13681-13691;
(b) H.M. Li, K.M. Belyk, J.J. Yin, et al., J. Am. Chem. Soc. 137 (2015) 13728-13731;
(c) J.L. Wood, B.M. Stoltz, S.N. Goodman, K. Onwueme, J. Am. Chem. Soc. 119 (1997)96529661.
-
[14]
(a) Y. Zhou, W.B. Cao, L.L. Zhang, X.P. Xu, S.J. Ji, J. Org. Chem. 83 (2018) 6056-6065;
(b) Y. Zhou, X.P. Xu, S.J. Ji, Org. Lett. 21 (2019) 2039-2042;
(c) R.Y. Zhang, M.M. Xu, H.Y. Li, X.P. Xu, S.J. Ji, Chin. Chem. Lett. 32 (2021) 433-436.
-
[15]
(a) T. Kagayama, A. Nakano, S. Sakaguchi, Y. Ishil, Org. Lett. 8 (2006) 407-409;
(b) D. Ryzhakov, M. Jarret, J.P. Baltaze, et al., Org. Lett. 21 (2019) 4986-4990;
(c) Y.Z. Gao, X.Q. Li, J. Xu, et al., Chem. Commun. 51 (2015) 1605-1607;
(d) Y.Z. Gao, J. Wu, J. Xu, et al., RSC Adv. 4 (2014) 51776-51779;
(e) J.F. Xue, S.F. Zhou, Y.Y. Liu, et al., Org. Biomol. Chem. 13 (2015) 4896-4902;
(f) L. Li, J.J. Wang, G.W. Wang, J. Org. Chem. 81 (2016) 5433-5439;
(g) M. Mondal, U. Bora, RSC Adv. 3 (2013) 18716-18754;
(h) I.S. Hong, J. Suh, Org. Lett. 2 (2000) 377-380;
(i) W.C. Yang, B. Li, M.M. Zhang, et al., Chin. Chem. Lett. 31 (2020) 1313-1316.

 Login In
Login In






 DownLoad:
DownLoad: