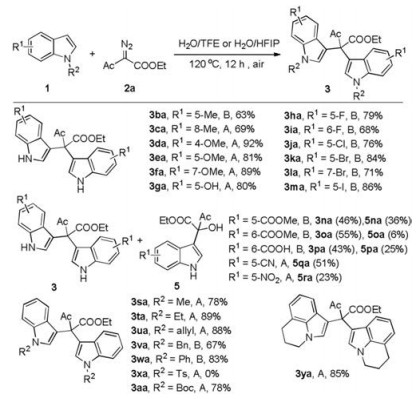
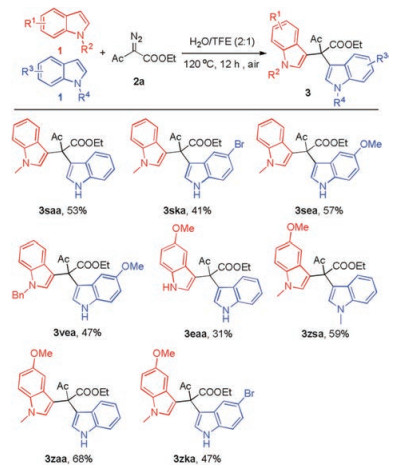
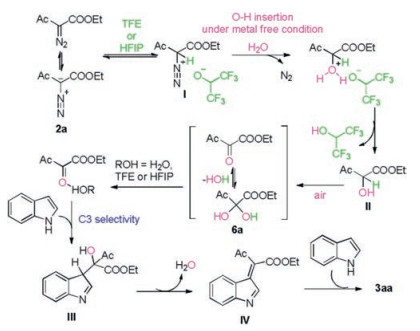
-
[1]
(a) T.R. Garbe, M. Kobayashi, N. Shimizu, et al., J. Nat. Prod. 63 (2000) 596-598;
(b) M. Kobayashi, S. Aoki, K. Gato, et al., Chem. Pharm. Bull. 42 (1994) 2449-2451;
(c) R. Veluri, I. Oka, I. Wagner-Döbler, H. Laatsch, J. Nat. Prod. 66 (2003) 1520-1523;
(d) R. Bell, S. Carmeli, N. Sar, J. Nat. Prod. 57 (1994) 1587-1590.
-
[2]
(a) C. Hong, G.L. Firestone, L.F. Bjeldanes, Biochem. Pharmacol. 63 (2002) 1085-1097;
(b) T. Irie, K. Kubushiro, K. Suzuki, et al., Anticancer Res. 19 (1999) 3061-3066;
(c) H.T. Le, C.M. Schaldach, G.L. Firestone, L.F. Bjeldanes, J. Biol. Chem. 278 (2003) 21136-21145;
(d) A. Kamal, Y.V.V. Srikanth, M.N.A. Khan, T.B. Shaik, M. Ashraf, Bioorg. Med. Chem. Lett. 20 (2010) 5229-5231;
(e) B.V. Subba Reddy, N. Rajeswari, M. Sarangapani, et al., Bioorg. Med. Chem. Lett. 22 (2012) 2460-2463;
(f) M. Shiri, M.A. Zolfigol, H.G. Kruger, Z. Tanbakouchian, Chem. Rev. 110 (2010) 2250-2293.
-
[3]
(a) G. Gao, Y. Han, Z.H. Zhang, ChemistrySelect 2 (2017) 11561-11564;
(b) J. Xiao, H. Wen, L. Wang, et al., Green Chem. 18 (2016) 1032-1037.
-
[4]
(a) K. Fuji, Chem. Rev. 93 (1993) 2037-2066;
(b) I. Denissova, L. Barriault, Tetrahedron 59 (2003) 10105-10146;
(c) K.W. Quasdorf, L.E. Overman, Nature 516 (2014) 181-191;
(d) R. Long, J. Huang, J. Gong, Z. Yang, Nat. Prod. Rep. 32 (2015) 1584-1601;
(e) X.P. Zeng, Z.Y. Cao, Y.H. Wang, F. Zhou, J. Zhou, Chem. Rev. 116 (2016) 7330-7396;
(f) J. Feng, M. Holmes, M.J. Krische, Chem. Rev. 117 (2017) 12564-12580;
(g) Y. Li, S. Xu, Chem. Eur. J. 24 (2018) 16218-16245;
(h) C. Li, S.S. Ragab, G. Liu, W. Tang, Nat. Prod. Rep. 37 (2020) 276-292.
-
[5]
(a) M. Delgado-Rebollo, A. Prieto, P.J. Pérez, ChemCatChem 6 (2014) 2047-2052;
(b) M.B. Johansen, M.A. Kerr, Org. Lett. 12 (2010) 4956-4959;
(c) A. DeAngelis, V.W. Shurtleff, O. Dmitrenko, J.M. Fox, J. Am. Chem. Soc. 133 (2011) 1650-1653;
(d) X. Gao, B. Wu, W.X. Huang, M.W. Chen, Y.G. Zhou, Angew. Chem. Int. Ed. 54 (2015) 11956-11960;
(e) V. Arredondo, S.C. Hiew, E.S. Gutman, I.D.U.A. Premachandra, D.L. Van Vranken, Angew. Chem. Int. Ed. 56 (2017) 4156-4159.
-
[6]
Z. Du, Y. Xing, P. Lu, Y. Wang, Org. Lett. 17(2015) 1192-1195.
doi: 10.1021/acs.orglett.5b00089
-
[7]
R.R. Singh, R.S. Liu, Chem. Commun. 53(2017) 4593-4596.
doi: 10.1039/C7CC01304C
-
[8]
X. Hu, F. Chen, Y. Deng, H. Jiang, W. Zeng, Org. Lett. 20(2018) 6140-6143.
doi: 10.1021/acs.orglett.8b02613
-
[9]
(a) C.J. Li, Chem. Rev. 105 (2005) 3095-3166;
(b) C.J. Li, L. Chen, Chem. Soc. Rev. 35 (2006) 68-82;
(c) A. Chanda, V.V. Fokin, Chem. Rev. 109 (2009) 725-748;
(d) R.N. Butler, A.G. Coyne, Chem. Rev. 110 (2010) 6302-6337;
(e) M.B. Gawande, V.D.B. Bonifácio, R. Luque, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42 (2013) 5522-5551.
-
[10]
(a) R. Breslow, Acc. Chem. Res. 24 (1991) 159-164;
(b) R. Breslow, Acc. Chem. Res. 37 (2004) 471-478;
(c) S. Narayan, J. Muldoon, M.G. Finn, et al., Angew. Chem. Int. Ed. 44 (2005) 3275-3279;
(d) W. Li, G. Yin, L. Huang, et al., Green Chem. 18 (2016) 4879-4883;
(e) C. Wu, X. Xin, Z.M. Fu, et al., Green Chem. 19 (2017) 1983-1989;
(f) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290;
(g) Y. Kim, C.J. Li, Green Synth. Catal. 1 (2020) 1-11.
-
[11]
(a) J.P. Bégué, D. Bonnet-Delpon, B. Crousse, Synlett (2004) 18-29;
(b) I.A. Shuklov, N.V. Dubrovina, A. Börner, Synthesis (2007) 2925-2943;
(c) I. Colomer, A.E.R. Chamberlain, M.B. Haughey, T.J. Donohoe, Nat. Rev. Chem. 1 (2017) 0088;
(d) J. Wencel-Delord, F. Colobert, Org. Chem. Front. 3 (2016) 394-400.
-
[12]
(a) H.F. Motiwala, R.H. Vekariya, J. Aubé, Org. Lett. 17 (2015) 5484-5487;
(b) R.H. Vekariya, J. Aubé, Org. Lett. 18 (2016) 3534-3537;
(c) G.X. Li, J. Qu, Chem. Commun. 46 (2010) 2653-2655;
(d) M. De Rosa, A. Soriente, Eur. J. Org. Chem. (2010) 1029-1032;
(e) Z. Sadiq, M. Iqbal, E.A. Hussain, S. Naz, J. Mol. Liq. 255 (2018) 26-42;
(f) W.C. Shiyao Lu, Qilong Shen, Chin. Chem. Lett. 30 (2019) 2279-2281.
-
[13]
M.B. Rubin, R. Gleiter, Chem. Rev. 100(2000) 1121-1164.
doi: 10.1021/cr960079j
-
[14]
A. Saba, Synth. Commun. 24(1994) 695-699.
doi: 10.1080/00397919408012648
-
[15]
Y. Yu, Q. Sha, H. Cui, K.S. Chandler, M.P. Doyle, Org. Lett. 20(2018) 776-779.
doi: 10.1021/acs.orglett.7b03912
-
[16]
(a) R.D.C. Gallo, A.C.B. Burtoloso, Green Chem. 20 (2018) 4547-4556;
(b) H.H. San, S.J. Wang, M. Jiang, X.Y. Tang, Org. Lett. 20 (2018) 4672-4676.
-
[17]
(a) S.F. Zhu, C. Chen, Y. Cai, Q.L. Zhou, Angew. Chem. Int. Ed. 47 (2008) 932-934;
(b) S.F. Zhu, Y. Cai, H.X. Mao, J.H. Xie, Q.L. Zhou, Nat. Chem. 2 (2010) 546-551.
-
[18]
X.J. Lv, Y.H. Chen, Y.K. Liu, Org. Lett. 21(2019) 190-195.
doi: 10.1021/acs.orglett.8b03654
-
[19]
B.M. Trost, S. Malhotra, P. Koschker, P. Ellerbrock, J. Am. Chem. Soc. 134(2012) 2075-2084.
doi: 10.1021/ja206995s
-
[20]
(a) D. Vuluga, J. Legros, B. Crousse, et al., J. Org. Chem. 76 (2011) 1126-1133;
(b) S. Khaksar, S.M. Vahdat, M. Gholizadeh, S.M. Talesh, J. Fluorine Chem. 136 (2012) 8-11;
(c) C. Pi, X. Yin, X. Cui, Y. Ma, Y. Wu, Org. Lett. 21 (2019) 2081-2084.

 Login In
Login In






 DownLoad:
DownLoad: