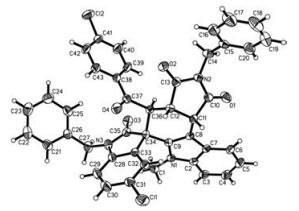
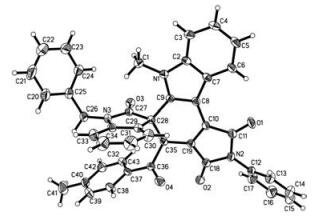
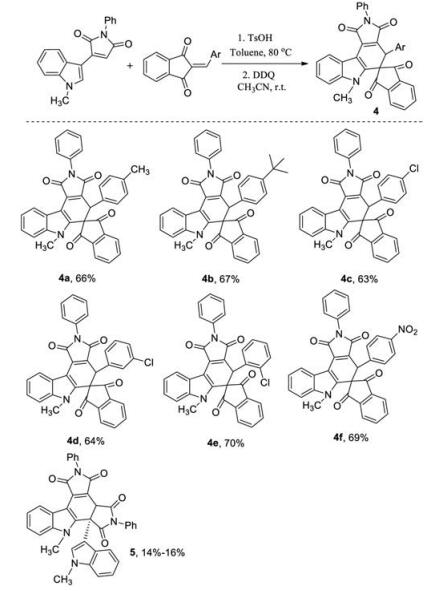
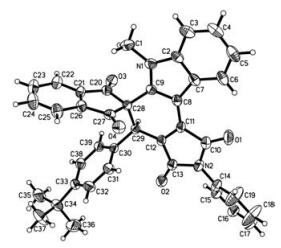
-
[1]
(a) L.T. Shen, P.L. Shao, S. Ye, Adv. Synth. Catal. 353 (2011) 1943-1948;
(b) T.Z. Li, Y. Jiang, Y.Q. Guan, et al., Chem. Commun. 50 (2014) 10790-10792;
(c) T.P. Gao, J.B. Lin, X.Q. Hu, et al., Chem. Commun. 50 (2014) 8934-8936;
(d) Y. Que, T. Li, C. Yu, et al., Org. Chem. 80 (2015) 3289-3294.
-
[2]
(a) G.J. Mei, F. Shi, Chem. Commun. 54 (2018) 6607-6621;
(b) G.J. Mei, D. Li, G.X. Zhou, et al., Chem. Commun. 53 (2017) 10030-10033;
(c) J.L. Wu, B.X. Du, Y.C. Zhang, et al., Adv. Synth. Catal. 358 (2016) 2777-2790;
(d) Q.N. Zhu, Y.C. Zhang, M.M. Xu, et al., J. Org. Chem. 81 (2016) 7898-7907.
-
[3]
(a) K. Thevissen, A. Marchand, P. Chaltin, et al., Curr. Med. Chem. 16 (2009) 2205-2211;
(b) R. Hesse, O. Kataeva, A.W. Schmidt, et al., Chem. Eur. J. 20 (2014) 9504-9509;
(c) M.S. Shaikh, R. Karpoormath, N. Thapliyal, et al., Anticancer Agents Med. Chem. 15 (2015) 1049-1065;
(d) S.P. Zhu, W.Y. Wang, K. Fang, et al., Chin. Chem. Lett. 25 (2014) 229-233.
-
[4]
(a) H.J. Jiang, J. Sun, J.L. Zhang, Curr. Org. Chem. 16 (2012) 2014-2025;
(b) K.T. Kamtekar, A.P. Monkman, M.R. Bryce, Adv. Mater. 22 (2010) 572-582;
(c) M.K. Hong, M.K. Ravva, P. Winget, et al., Chem. Mater. 28 (2016) 5791-5798;
(d) B.W. Li, X.A. Song, X. Jiang, et al., Chin. Chem. Lett. 31 (2020) 1188-1192;
(e) Y. Xiong, J.J. Zeng, et al., Chin. Chem. Lett. 30 (2019) 592-596.
-
[5]
(a) H.J. Knolker, R.R. Kethiri, Chem. Rev. 102 (2002) 4303-4427;
(b) A.W. Schmidt, K.R. Reddy, H.J. Knlker, Chem. Rev. 112 (2012) 3193-3328;
(c) S. Lancianesi, A. Palmieri, M. Petrini, Chem. Rev. 114 (2014) 7108-7149;
(d) S.Z. Zhao, R.B. Andrade, J. Am. Chem. Soc. 135 (2013) 13334-13337;
(e) T.L. Lan, H.J. Qin, W.T. Chen, et al., Chin. Chem. Lett. 31 (2020) 357-360.
-
[6]
(a) V.P. Kumar, K.K. Gruner, O. Kataeva, et al., Angew. Chem. Int. Ed. 52 (2013) 11073-11077;
(b) S.H. Cho, J. Yoon, S. Chang, J. Am. Chem. Soc. 133 (2011) 5996-6005;
(c) A.C. Hernandez-Perez, S.K. Collins, Angew. Chem. Int. Ed. 52 (2013) 12696-12700;
(d) H. Gao, Q.L. Xu, M. Yousufuddin, et al., Angew. Chem. Int. Ed. 53 (2014) 2701-2705.
-
[7]
(a) C. Liu, X.Q. Han, X. Wang, et al., J. Am. Chem. Soc. 126 (2004) 3700-3701;
(b) N. Kuroda, Y. Takah ashi, K. Yoshinaga, et al., Org. Lett. 8 (2006) 1843-1845;
(c) F. Zhao, N. Li, Y.F. Zhu, et al., Org. Lett. 18 (2016) 1506-1509;
(d) S.Z. Zhao, R.B. Andrade, J. Org. Chem. 82 (2017) 521-531.
-
[8]
(a) C. Gioia, A. Hauville, L. Bernardi, et al., Angew. Chem. Int. Ed. 47 (2008) 9236-9239;
(b) Y. Tao, F. Zhang, C.Y. Tang, et al., Asian J. Org. Chem. 3 (2014) 1292-1301;
(c) L.J. Zhou, B. Xu, J.L. Zhang, Angew. Chem. Int. Ed. 54 (2015) 9092-9096;
(d) J.W. Ren, Z.F. Zhou, J.A. Xiao, et al., Eur. J. Org. Chem. 7 (2016) 1264-1268.
-
[9]
(a) Y.T. Yang, J.F. Zhu, G.C. Liao, et al., Med. Chem. 25 (2018) 2233-2244;
(b) N. Ye, H.Y. Chen, E.A. Wold, et al., ACS Infect. Dis. 2 (2016) 382-392;
(c) B. Yu, D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97 (2015) 673-698;
(d) B. Yu, Z.Q. Yu, P.P. Qi, et al., Eur. J. Med. Chem. 95 (2015) 35-40.
-
[10]
(a) G.S. Singh, Z.Y. Desta, Chem. Rev. 112 (2012) 6104-6155;
(b) L. Hong, R. Wang, Adv. Synth. Catal. 355 (2013) 1023-1052;
(c) Y. Liu, H. Wang, J. Wan, Asian J. Org. Chem. 2 (2013) 374-386;
(d) Z. Liu, N. Li, X. Huang, et al., Tetrahedron. 70 (2014) 2406-2415.
-
[11]
(a) B. Tan, G. Hernandez-Torres, C.F. Barbas, J. Amer. Chem. Soc. 133 (2011) 12354-12357;
(b) H.F. Zheng, P. He, Y.B. Liu, et al., Chem. Commun. 50 (2014) 8794-8796;
(c) P. Sharma, N.P. Kumar, N.H. Krishna, et al., Org. Chem. Front. 3 (2016) 1503-1508;
(d) Z.H. You, Y.H. Chen, Y. Tang, et al., Org. Lett. 20 (2018) 6682-6686.
-
[12]
(a) Y.K. Liu, M. Nappi, E. Arceo, et al., J. Am. Chem. Soc. 133 (2011) 15212-15218;
(b) Y. Wang, M.S. Tu, L. Yin, et al., J. Org. Chem. 80 (2015) 3223-3232;
(c) J.W. Ren, J. Wang, J.A. Xiao, et al., J. Org. Chem. 82 (2017) 6441-6449;
(d) L.J. Huang, J. Weng, S. Wang, et al., Adv. Synth. Catal. 357 (2015) 993-1003.
-
[13]
(a) R.Y. Yang, J. Sun, Y. Tao, et al., J. Org. Chem. 82 (2017) 13277-13287;
(b) R.Y. Yang, J. Sun, Q. Sun, C.G. Yan, J. Org. Chem. 83 (2018) 5909-5919;
(c) D.Q. Wang, J. Sun, C.G. Yan, Chemistryselect 4 (2019) 10550-10554;
(d) J. Sun, R.Y. Yang, S.C. Zhan, et al., ChemistrySelect 4 (2019) 10100-10103;
(e) R. Ye, C.G. Yan, Eur. J. Org. Chem (2019) 5882-5886;
(f) S.C. Zhan, J. Sun, R.Z. Liu, et al., Org. Biomol. Chem. 18 (2020) 163-168.
-
[14]
(a) Y.L. An, Z.Y. Shao, J. Chen, et al., Synthesis 45 (2013) 2719-2726;
(b) E. Pereira, A. Youssef, M. El-Ghozzi, et al., Tetrahedron Lett. 55 (2014) 834-837.
-
[15]
(a) H. Henon, S. Messaoudi, B. Hugon, et al., Tetrahedron 61 (2005) 5599-5614;
(b) E. Conchon, F. Anizon, B. Aboab, et al., Bioorg. Med. Chem. 16 (2008) 4419-4430;
(c) B. Hugon, B. Pfeiffer, P. Renard, et al., Tetrahedron Lett. 44 (2003) 3935-3937;
(d) E. Conchon, F. Anizon, B. Aboa, et al., J. Med. Chem. 50 (2007) 4669-4680;
(e) H. Hénon, F. Anizon, N. Kucharczyk, et al., Synthesis (2006) 711-715;
(f) Y.L. An, Z.H. Yang, H.H. Zhang, et al., Org. Lett. 18 (2016) 152-155.
-
[16]
(a) J. Sun, Y. Sun, H. Gong, et al., Org. Lett. 14 (2012) 5172-5175;
(b) J. Sun, Y.J. Xie, C.G. Yan, J. Org. Chem. 78 (2013) 8354-8365;
(c) H. Gao, J. Sun, C.G. Yan, J. Org. Chem. 79 (2014) 4131-4136;
(d) Y. Han, Y.J. Sheng, C.G. Yan, Org. Lett. 16 (2014) 2654-2657;
(e) J. Sun, L. Chen, H. Gong, et al., Org. Biomol. Chem. 13 (2015) 5905-5917;
(f) L. Chen, J. Sun, J. Xie, et al., Org. Biomol. Chem. 14 (2016) 6497-6507.
-
[17]
(a) R.G. Shi, X.H. Wang, C.G. Yan, et al., Chem. Commun. 52 (2016) 6280-6283;
(b) J. Cao, J. Sun, C.G. Yan, Org. Biomol. Chem. 16 (2018) 4170-4175;
(c) R.Z. Liu, R.G. Shi, J. Sun, et al., Org. Chem. Front. 4 (2017) 354-357;
(d) J. Sun, Y. Zhang, R.G. Shi, et al., Org. Biomol. Chem. 17 (2019) 3978-3983;
(e) J. Cao, F. Yang, J. Sun, et al., J. Org. Chem. 84 (2019) 622-635.

 Login In
Login In






 DownLoad:
DownLoad: