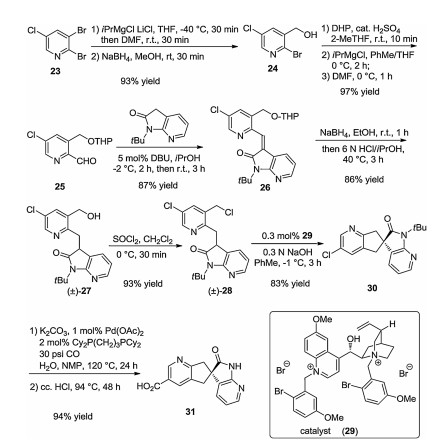
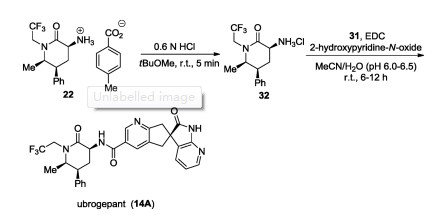
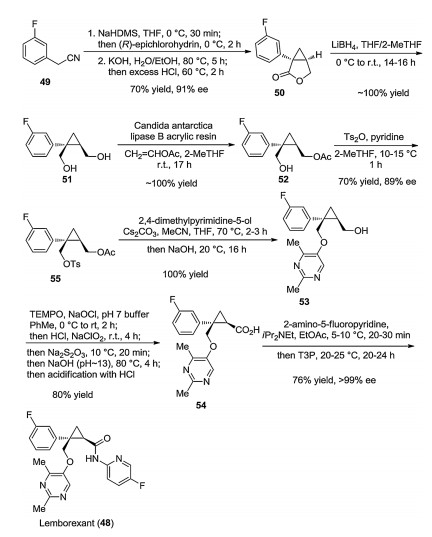
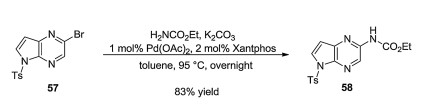
-
[1]
(a) J. Fried, E.F. Sabo, J. Am. Chem. Soc. 75 (1953) 2273-2274;
(b) J. Fried, E.F. Sabo, J. Am. Chem. Soc. 76 (1954) 1455-1456.
-
[2]
(a) C. Heidelberger, N.K. Chaudhuri, P. Danneberg, et al., Nature 179 (1957) 663-666;
(b) D.B. Longley, D.P. Harkin, P.G. Johnston, Nat. Rev. Cancer 3 (2003) 330-338.
-
[3]
D. O'Hagan, J. Fluorine Chem. 131 (2010) 1071-1081.
doi: 10.1016/j.jfluchem.2010.03.003
-
[4]
J.A. Tobert, Nat. Rev. Drug Discov. 2 (2003) 517-526.
doi: 10.1038/nrd1112
-
[5]
(a) Q.A. Huchet, B. Kuhn, B. Wagner, et al., J. Med. Chem. 58 (2015) 9041-9060;
(b) B. Jeffries, Z. Wang, H.R. Felstead, et al., J. Med. Chem. 63 (2020) 1002-1031;
(c) A.D. Wade, A. Rizzi, Y. Wang, D.J. Huggins, J. Chem. Inf. Model. 59 (2019) 2776-2784;
(d) E.N.G. Marsh, Acc. Chem. Res. 47 (2014) 2878-2886;
(e) A.A. Berger, J.S. Völler, N. Budisa, B. Koksch, Acc. Chem. Res. 50 (2017) 2093-2103;
(f) L. Kiss, A.M. Remete, Eur. J. Org. Chem. (2019) 5574-5602;
(g) L. Kiss, F. Fülöp, Chem. Rec. (2018) 266-281;
(h) A.M. Remete, M. Nonn, S. Fustero, F. Fülöp, L. Kiss, Tetrahedron 74 (2018) 6367-6418;
(i) M. Nonn, L. Kiss, M. Haukka, S. Fustero, F. Fülöp, Org. Lett. 17 (2015) 1074-1077;
(j) J. Liu, Z. Li, H. Mei, V.A. Soloshonok, J.L. Han, ACS Omega 4 (2019) 19505-19512;
(k) C. Xie, L. Zhang, W. Sha, et al., Org. Lett. 18 (2016) 3270-3273;
(l) X. Xu, X. Dong, Z. Zhang, et al., Macroheterocycles 12 (2019) 403-408.
-
[6]
(a) J. Wang, M. Sánchez-Roselló, J.L. Aceña, et al., Chem. Rev. 114 (2014) 2432-2506;
(b) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J.L. Aceña, V.A. Soloshonok, K. Izawa, H. Liu, Chem. Rev. 116 (2016) 422-518;
(c) W. Zhu, J. Wang, S. Wang, et al., J. Fluorine Chem. 167 (2014) 37-54;
(d) Y. Zhu, J.L. Han, J. Wang, et al., Chem. Rev. 118 (2018) 3887-3964;
(e) H. Mei, J. Han, K.D. Klika, et al., Eur. J. Med. Chem. 186 (2020) 111826;
(f) N.A. Meanwell, J. Med. Chem. 61 (2018) 5822-5880.
-
[7]
H. Mei, J. Han, S. Fustero, et al., Chem. Eur. J. 25 (2019) 11797-11819.
doi: 10.1002/chem.201901840
-
[8]
H.A. Blair, Drugs 80 (2020) 417-423.
doi: 10.1007/s40265-020-01271-6
-
[9]
A.J. Robichaud, T. Lee, W. Deng, et al., PCT/US2000/016498, 2000.
-
[10]
T. Lee, A.J. Robichaud, K.E. Boyle, et al., Bioorg. Med. Chem. Lett. 13 (2003) 767-770.
doi: 10.1016/S0960-894X(02)01028-4
-
[11]
P. Li, Q. Zhang, A.J. Robichaud, et al., J. Med. Chem. 57 (2014) 2670-2682.
doi: 10.1021/jm401958n
-
[12]
R.E. Davis, C.U. Correll, Expert Rev. Neurother. 16 (2016) 601-614.
doi: 10.1080/14737175.2016.1174577
-
[13]
N. Zisapel, Expert Opin, Investig. Drugs 24 (2014) 401-411.
-
[14]
J.A. Lieberman, R.E. Davis, C.U. Correll, et al., Biol. Psychiatry 79 (2016) 952-961.
doi: 10.1016/j.biopsych.2015.08.026
-
[15]
K.E. Vanover, R.E. Davis, Y. Zhou, et al., Neuropsychopharmacology 44 (2019) 598-605.
doi: 10.1038/s41386-018-0251-1
-
[16]
F. Carponi, C. Fabbri, I. Bitter, et al., Eur. Neuropsychopharmacol. 29 (2019) 971-985.
doi: 10.1016/j.euroneuro.2019.06.008
-
[17]
I.M. Bell, M.E. Fraley, S.N. Gallicchio, et al., US2010/0122899, 2012.
-
[18]
P.R. Holland, P.J. Goadsby, Neurotherapeutics 15 (2018) 304-312.
doi: 10.1007/s13311-018-0617-4
-
[19]
P. Martelletti, M.A. Giamberardino, Expert Opin. Pharmacol. 20 (2018) 209-218.
-
[20]
R.B. Lipton, D.W. Dodick, J. Ailani, et al., JAMA 322 (2019) 1887-1898.
doi: 10.1001/jama.2019.16711
-
[21]
I.M. Bell, M.E. Fraley, S.N. Gallicchio, et al., PCT/US2011/060081, 2012.
-
[22]
F. Chen, C. Molinaro, W.P. Wuelfing, et al., PCT/US2013/030692, 2013.
-
[23]
K.M. Belyk, E. Cleator, S.C. Kuo, et al., PCT/US2013/030696, 2013.
-
[24]
N. Yasuda, E. Cleator, B. Kosjek, et al., Org. Process Res. Dev. 21 (2017) 1851-1858.
doi: 10.1021/acs.oprd.7b00293
-
[25]
P. Gergely, B. Nuesslein-Hildesheim, D. Guerini, et al., Brit. J. Pharmacol. 167 (2012) 1035-1047.
doi: 10.1111/j.1476-5381.2012.02061.x
-
[26]
S. Pan, N.S. Gray, W. Gao, et al., ACS Med. Chem. Lett. 4 (2013) 333-337.
doi: 10.1021/ml300396r
-
[27]
E. Legangneux, A. Gardin, D. Johns, Br. J. Clin. Pharmacol. 75 (2012) 831-841.
-
[28]
K. Selmaj, D.K.B. Li, H.P. Hartung, et al., Lancet Neurol. 12 (2013) 756-767.
doi: 10.1016/S1474-4422(13)70102-9
-
[29]
R.M. Fryer, A. Muthukumarana, P.C. Harrison, et al., PLoS One 7 (2012) e52985.
doi: 10.1371/journal.pone.0052985
-
[30]
Z.T. Al-Salama, Drugs 79 (2019) 1009-1015.
doi: 10.1007/s40265-019-01140-x
-
[31]
L. Kappos, A. Bar-Or, B.A.C. Cree, Lancet 391 (2018) 1263-1273.
doi: 10.1016/S0140-6736(18)30475-6
-
[32]
U. Glaenzel, Y. Jin, R. Nufer, et al., Drug Metab. Dispos. 46 (2018) 1001-1013.
doi: 10.1124/dmd.117.079574
-
[33]
A. Gentile, A. Musella, S. Bullitta, et al., J. Neuroinflamm. 13 (2016) 207.
doi: 10.1186/s12974-016-0686-4
-
[34]
C. O'Sullivan, A. Schubart, A.K. Mir, K.K. Dev, J. Neuroinflamm. 13 (2016) 31.
doi: 10.1186/s12974-016-0494-x
-
[35]
R. Yamamoto, T. Aoki, H. Koseki, et al., Br. J. Pharmacol. 174 (2017) 2085-2101.
doi: 10.1111/bph.13820
-
[36]
Z. Chen, T.M. Doyle, L. Luongo, et al., PNAS 116 (2019) 10557-10562.
doi: 10.1073/pnas.1820466116
-
[37]
T. Bobinger, A. Manaenko, P. Burkardt, et al., Stroke 50 (2019) 3246-3254.
doi: 10.1161/STROKEAHA.119.027134
-
[38]
S. Pan, W. Gao, N.S. Gray, Y. Mi, Y. Fan, PCT/US2004/015603, 2004.
-
[39]
Y. Liu, D. Papoutsakis, E. Roddy, PCT/US2009/068346, 2010.
-
[40]
L. Ciszewski, M. de la Cruz, P.H. Karpinski, et al., PCT/US2009/068143, 2010.
-
[41]
M. de la Cruz, P.H. Karpinski, Y. Liu, PCT/US2009/068352, 2010.
-
[42]
F. Gallou, J.M. Sedelmeier, C. Vogel, PCT/EP2013/052106, 2013.
-
[43]
Y. Yoshida, T. Terauchi, Y. Naoe, et al., Bioorg. Med. Chem. 22 (2014) 6071-6088.
doi: 10.1016/j.bmc.2014.08.034
-
[44]
Y. Yoshida, Y. Naoe, T. Terauchi, et al., J. Med. Chem. 58 (2015) 4648-4664.
doi: 10.1021/acs.jmedchem.5b00217
-
[45]
P.J. Murphy, S. Yasuda, K. Nakai, et al., J. Clin. Pharmacol. 57 (2017) 96-104.
doi: 10.1002/jcph.785
-
[46]
P. Murphy, M. Moline, D. Mayleben, et al., J. Clin. Sleep Med. 13 (2017) 1289-1299.
doi: 10.5664/jcsm.6800
-
[47]
R. Rosenberg, P. Murphy, G. Zammit, et al., JAMA Netw. Open 2 (2019) e1918254.
doi: 10.1001/jamanetworkopen.2019.18254
-
[48]
G.A. Moniz, A.Z. Wilcoxen, F. Benayoud, et al., PCT/US2013/026204, 2013.
-
[49]
S. Nakayamada, S. Kubo, S. Iwata, Y. Tanaka, BioDrugs 30 (2016) 407-419.
doi: 10.1007/s40259-016-0190-5
-
[50]
M.E.F. Mohamed, H.S. Camp, P. Jiang, et al., Clin. Pharmacokinet. 55 (2016) 1547-1558.
doi: 10.1007/s40262-016-0419-y
-
[51]
N. Wishart, M.A. Argiriadi, D.J. Calderwood, et al., WO2011068881, 2011.
-
[52]
(a) G.R. Burmester, J.M. Kremer, F. Van den Bosch, et al., Lancet 391 (2018) 2503-2512;
(b) M.C. Genovese, R. Fleischmann, B. Combe, et al., Lancet 391 (2018) 2513-2524.
-
[53]
J.S. Smolen, A.L. Pangan, P. Emery, et al., Lancet 393 (2019) 2303-2311.
doi: 10.1016/S0140-6736(19)30419-2
-
[54]
R. Fleischmann, A.L. Pangan, I.H. Song, et al., Arthritis Rheumatol. 71 (2019) 1788-1800.
doi: 10.1002/art.41032
-
[55]
F. D'Amico, G. Fiorino, F. Furfaro, M. Allocca, S. Danese, Expert Opin. Invest. Drugs 27 (2018) 595-599.
doi: 10.1080/13543784.2018.1492547
-
[56]
T. Perez-Jeldres, C.J. Tyler, J.D. Boyer, et al., Front. Pharmacol. 10 (2019) 212.
doi: 10.3389/fphar.2019.00212
-
[57]
R. Panaccione, G.R. D'Haens, W.J. Sandborn, et al., Gastroenterology 156 (2019) S-170.
-
[58]
E. Guttman-Yassky, D. Thaçi, A.L. Pangan, et al., J. Allergy Clin. Immunol. (2019) 145 (2020) 877-884.
doi: 10.1016/j.jaci.2019.11.025
-
[59]
N. Wishart, M.A. Argiriadi, D.J. Calderwood, PCT/US2010/058572, 2011.
-
[60]
J.W. Voss, H.S. Camp, R.J. Padley, PCT/US2014/062145, 2015.
-
[61]
(a)V.A. Soloshonok, H. Ohkura, K.Uneyama, Tetrahedron Lett. 43 (2002) 5449-5452;
(b) V.A. Soloshonok, T. Ono, I.V. Soloshonok, J. Org. Chem. 62 (1997) 7538-7539;
(c) V.A. Soloshonok, V.P. Kukhar, Tetrahedron 52 (1996) 6953-6964.
-
[62]
A. Allian, J. Jayanth, M.E. Mohamed, et al., PCT/US2016/057372, 2017.
-
[63]
S. Oruganti, B. Kandagatla, S. Sen, et al., PCT/IB2018/055368, 2019.
-
[64]
R. Singh, U. Manjunatha, H.I.M. Boshoff, et al., Science 322 (2008) 1392-1395.
doi: 10.1126/science.1164571
-
[65]
S. Patterson, S. Wyllie, L. Stojanovski, et al., Antimicrob. Agents Chemother. 57 (2013) 4699-4706.
doi: 10.1128/AAC.00722-13
-
[66]
(a) M.A. Marsini, P.J. Reider, E.J. Sorensen, J. Org. Chem. 75 (2010) 7479-7482;
(b) G.C. Moraski, A.G. Oliver, L.D. Markley, et al., Bioorg. Med. Chem. Lett. 24 (2014) 3493-3498.
-
[67]
S.J. Keam, Drug 79 (2019) 1797-1803.
doi: 10.1007/s40265-019-01207-9
-
[68]
W.R. Baker, C. Shaopei, E.L. Keeler, US 6087358, 2000.
-
[69]
P. Furet, V. Guagnano, R.A. Fairhurst, et al., Bioorg. Med. Chem. Lett. 23 (2013) 3741-3748.
doi: 10.1016/j.bmcl.2013.05.007
-
[70]
M. Gerspacher, R.A. Fairhurst, R. Mah, et al., Bioorg. Med. Chem. Lett. 25 (2015) 3582-3584.
doi: 10.1016/j.bmcl.2015.06.077
-
[71]
F. André, E. Ciruelos, G. Rubovszky, et al., N. Engl. J. Med. 380 (2019) 1929-1940.
doi: 10.1056/NEJMoa1813904
-
[72]
A. Markham, Drugs 79 (2019) 1249-1253.
doi: 10.1007/s40265-019-01161-6
-
[73]
(a) V.P. Sandanayaka, S. Shacham, D. Mccauley, S. Shechter, WO2013019548, 2013;
(b) M. Garg, D. Kanojia, A. Mayakonda, et al., Oncotarget 8 (2017) 7521-7532
-
[74]
Y.Y. Syed, Drugs 79 (2019) 1485-1494.
doi: 10.1007/s40265-019-01188-9
-
[75]
A.R. Muthusamy, S.L. Kanniah, A. Ravi, et al., WO2018129227, 2018.
-
[76]
M. Menichincheri, E. Ardini, P. Magnaghi, et al., J. Med. Chem. 59 (2016) 3392-3408.
doi: 10.1021/acs.jmedchem.6b00064
-
[77]
D. Liu, M. Offin, S. Harnicar, B.T. Li, A. Drilon, Ther. Clin. Risk Manag. 14 (2018) 1247-1252.
doi: 10.2147/TCRM.S147381
-
[78]
R. Iyer, L. Wehrmann, R.L. Golden, et al., Cancer Lett. 372 (2016) 179-186.
doi: 10.1016/j.canlet.2016.01.018
-
[79]
G. Wei, R. Patel, C. Walsh, et al., Eur. J. Cancer 69 (2016) S33.
-
[80]
Z.T. AI-Salama, S.J. Keam, Drugs 79 (2019) 1477-1483.
doi: 10.1007/s40265-019-01177-y
-
[81]
C. Zhang, J. Zhang, P.N. Ibrahim, et al., WO2008064255, 2008.
-
[82]
P.N. Ibrahim, M. Jin, S. Matsuura, PCT Int. Appl. WO2016179412, 2016.
-
[83]
Y.N. Lamb, Drugs 79 (2019) 1805-1812.
doi: 10.1007/s40265-019-01210-0
-
[84]
D. Chen, Y. Zhang, J. Li, Y. Liu, Synthesis 51 (2019) 2564-2571.
doi: 10.1055/s-0037-1612421
-
[85]
D.L. Nelson, L.A. Phebus, K.W. Johnson, et al., Cephalalgia 30 (2010) 1159-1169.
doi: 10.1177/0333102410370873
-
[86]
M.D. Ferrari, M. Färkkilä, U. Reuter, et al., Cephalalgia 30 (2010) 1170-1178.
doi: 10.1177/0333102410375512
-
[87]
Y.J. Jin, X.W. Yang, X.X. Xie, H. Tian, Y.L. Cui, Drugs Clinic 31 (2016) 1300-1303.
-
[88]
Y.N. Lamb, Durgs 79 (2019) 1989-1996.
-
[89]
M.P. Cohen, D.T. Kohlman, S.X. Liang, V. Mancuso, F. Victor, PCT Int. Appl. WO200384949, 2003.
-
[90]
N.A. Meanwell, J. Med. Chem. 54 (2011) 2529-2591.
doi: 10.1021/jm1013693
-
[91]
(a) J.A. Ma, D. Cahard, J. Fluorine Chem. 128 (2007) 975-996;
(b) A. Sato, M.V. Ponomarenko, T. Ono, G.V. Röschenthaler, V.A. Soloshonok, Eur. J. Org. Chem. 2019 (2019) 4417-4421;
(c) H. Mei, J.L. Han, S. White, G. Butler, V.A. Soloshonok, J. Fluorine Chem. 227 (2019)109370;
(d) C. Yang, A. Hassanpour, K. Ghorbanpour, S. Abdolmohammadi, E. Vessally, RSC Adv. 9 (2019) 27625-27639;
(e) Z. Zhang, Adv. Synth. Catal. 359 (2017) 372-383;
(f) S.L. Clarke, G.P. McGlacken, Chem. Eur. J. 23 (2017) 1219-1230;
(g) Y. Ouyang, X.H. Xu, F.L. Qing, Angew. Chem. Int. Ed. 57 (2018) 6926-6929;
(h) J. Kalim, T. Duhail, T.N. Le, et al., Chem. Sci. 10 (2019) 10516-10523.
-
[92]
(a) J. Han, O. Kitagawa, A. Wzorek, K.D. Klika, V.A. Soloshonok, Chem. Sci. 9 (2018) 1718-1739;
(b) M.Yasumoto, H.Ueki, V.A.Soloshonok, J.FluorineChem.131 (2010)266-269.
-
[93]
(a) A.E. Sorochinsky, J.L. Aceña, V.A. Soloshonok, Synthesis 45 (2013) 141-152;
(b) M. Yasumoto, H. Ueki, T. Ono, T. Katagiri, V.A. Soloshonok, J. Fluorine Chem. 131 (2010) 535-539.
-
[94]
(a) A.E. Sorochinsky, T. Katagiri, T. Ono, et al., Chirality 25 (2013) 365-368;
(b) T. Nakamura, K. Tateishi, S. Tsukagoshi, et al., Tetrahedron 68 (2012) 4013-4017.
-
[95]
(a) H. Ueki, M. Yasumoto, V.A. Soloshonok, Tetrahedron Asymmetry 21 (2010) 1396-1400;
(b) J. Han, D.J. Nelson, A.E. Sorochinsky, V.A. Soloshonok, Curr. Org. Synth. 8 (2011) 310-317.
-
[96]
(a) C. Xie, L. Wu, J. Han, V.A. Soloshonok, Y. Pan, Angew. Chem. Int. Ed. 54 (2015) 6019-6023;
(b) J. Han, V.A. Soloshonok, K.D. Klika, J. Drabowicz, A. Wzorek, Chem. Soc. Rev. 47 (2018) 1307-1350.
-
[97]
(a) A. Henninot, J.C. Collins, J.M. Nuss, J. Med. Chem. 61 (2018) 1382-1414;
(b) M.A.T. Blaskovich, J. Med. Chem. 59 (2016) 10807-10836;
(c) D.R.W. Hodgson, J.M. Sanderson, Chem. Soc. Rev. 33 (2004) 422-430;
(d) T. Sato, K. Izawa, J.L. Aceña, H. Liu, V.A. Soloshonok, Eur. J. Org. Chem. 2016 (2016) 2757-2774;
(e) V.A. Soloshonok, K. Izawa, Asymmetric synthesis and application of α-amino acids, ACS Symposium Series 1009, Oxford University Press, Oxford, 2009;
(f) S. Wang, Y. Wang, J. Wang, et al., Curr. Pharm. Des. 23 (2017) 4493-4554.
-
[98]
(a) D. Stepec, M. Ponikvar-Svet, Acta Chim. Slovenica 66 (2019) 255-275;
(b) D. Stepec, G. Tavccar, M. Ponikvar-Svet, Environ. Pollut. 248 (2019) 958-964;
(c) M. Ponikvar, V. Stibilj, B. Zemva, Food Chem.103 (2007) 369-374;
(d) A. Koblar, G. Tavcar, M. Ponikvar-Svet, Food Chem.130 (2012) 286-290.

 Login In
Login In







 DownLoad:
DownLoad: