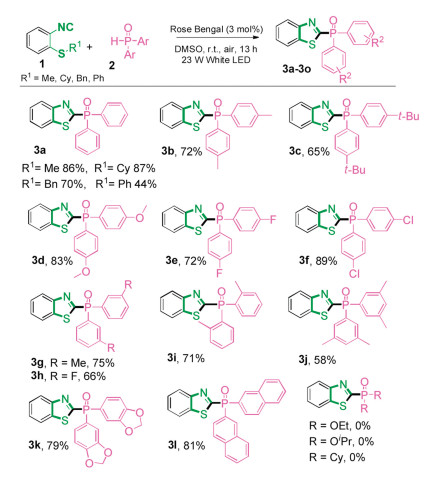
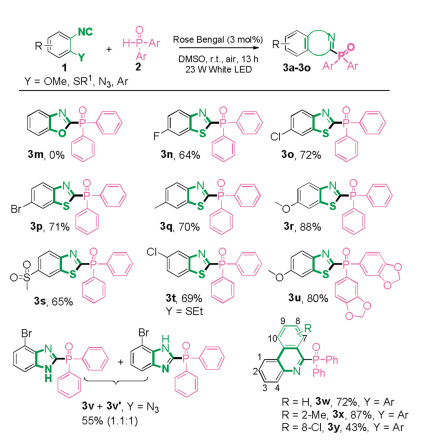
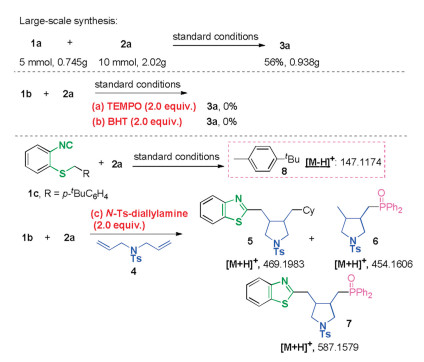
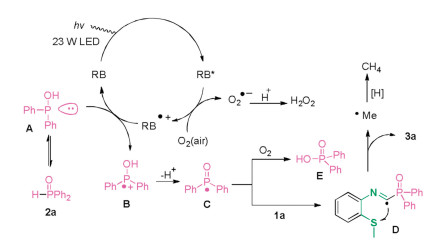
-
[1]
(a) J.L. Montchamp, Acc. Chem. Res. 47 (2013) 77-87;
(b) Queffélec, M. Petit, P. Janvier, D.A. Knight, B. Bujoli, Chem. Rev. 112 (2012) 3777-3807;
(c) C.S. Demmer, N. Krogsgaard-Larsen, L. Bunch, Chem. Rev. 111 (2011) 7981-8006;
(d) S. Van der Jeught, C.V. Stevens, Chem. Rev. 109 (2009) 2672-2702.
-
[2]
Y.N. Ma, S.X. Li, S.D. Yang, Acc. Chem. Res. 50(2017) 1480-1492.
doi: 10.1021/acs.accounts.7b00167
-
[3]
(a) M. Mao, L. Zhang, Y.Z. Chen, et al., ACS Catal. 7 (2017) 181-185;
(b) X. Mi, C. Wang, M. Huang, et al., Org. Lett. 16 (2014) 3356-3359;
(c) H.J. Zhang, W. Lin, Z. Wu, et al., Chem. Commun. 51 (2015) 3450-3453;
(d) R. Zhuang, J. Xu, S. Cai, et al., Org. Lett. 13 (2011) 2110-2113;
(e) M. Kalek, T. Johansson, M. Jezowska, et al., Org. Lett. 12 (2010) 4702-4704;
(f) D.Y. Hu, M.S. Li, W.W. Zhong, et al., Chin. Chem. Lett. 27 (2016) 1691-1695;
(g) Q. Fu, D. Yi, Z. Zhang, et al., Org. Chem. Front. 4 (2017) 1385-1389;
(h) M.S. Li, Q. Zhang, D.Y. Hu, et al., Tetrahedron Lett. 57 (2016) 2642-2646;
(i) Q. Huang, L. Zhu, D. Yi, et al., Chin. Chem. Lett. 31 (2020) 373-376;
(j) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368;
(k) X.C. Liu, K. Sun, X.L. Chen, et al., Adv. Synth. Catal. 361 (2019) 3712-3717;
(l) Q. Huang, K. Dong, W. Bai, et al., Org. Lett. 21 (2019) 3332-3336;
(m) Z.J. Zhang, D. Yi, Q. Fu, et al., Tetrahedron Lett. 58 (2017) 2417-2420;
(n) W. Liang, Z. Zhang, D. Yi, et al., Chin. J. Chem. 35 (2017) 1378-1382;
(o) C. Liu, M. Zhu, W. Wei, et al., Org. Chem. Front. 2 (2015) 1356-1360;
(p) W.W. Zhong, Q. Zhang, M.S. Li, et al., Synth. Commun. 46 (2016) 1377-1385;
(q) C. Liu, W. Wei, D. Yang, et al., Tetrahedron 71 (2015) 6901-6906;
(r) R. Li, X. Chen, S. Wei, et al., Adv. Synth. Catal. 360 (2018) 4807-4813.
-
[4]
(a) U. Wille, Chem. Rev. 113 (2013) 813-853;
(b) M.H. Muhammad, X.L. Chen, B. Yu, et al., Pure Appl. Chem. 91 (2018) 33-41;
(c) J. Zheng, Y. Zhang, D. Wang, et al., Org. Lett. 18 (2016) 1768-1771;
(d) Y. Gao, G. Lu, P. Zhang, et al., Org. Lett. 18 (2016) 1242-1245;
(e) H.L. Hua, B.S. Zhang, Y.T. He, et al., Org. Lett. 18 (2016) 216-219;
(f) Y.M. Li, M. Sun, H.L. Wang, et al., Angew. Chem. Int. Ed. 52 (2013) 3972-3976;
(g) Y. Zhang, J. Zhang, B. Hu, et al., Org. Lett. 20 (2018) 2988-2992;
(h) J. Xu, X. Yu, Q. Song, Org. Lett. 19 (2017) 980-983;
(i) W. Ren, Q. Yang, S.D. Yang, Pure Appl. Chem. 91 (2019) 87-94.
-
[5]
(a) N.A. Romero, D.A. Nicewicz, Chem. Rev. 116 (2016) 10075-10166;
(b) J.R. Chen, X.Q. Hu, L.Q. Lu, et al., Acc. Chem. Res. 49 (2016) 1911-1923;
(c) J.C. Tellis, C.B. Kelly, D.N. Primer, et al., Acc. Chem. Res. 49 (2016) 1429-1439;
(d) J. Twilton, C. Le, P. Zhang, et al., Nat. Rev. Chem. 1 (2017) 0052;
(e) L.Y. Xie, L.L. Jiang, J.X. Tan, et al., ACS Sustainable Chem. Eng. 7 (2019) 14153-14160;
(f) L.Y. Xie, T.G. Fang, J.X. Tan, et al., Green Chem. 21 (2019) 3858-3863;
(g) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1613;
(h) W. Wei, L. Wang, H. Yue, et al., ACS Sustainable Chem. Eng. 6 (2018) 17252-17257;
(i) W. Wei, P. Bao, H. Yue, et al., Org. Lett. 20 (2018) 5291-5295;
(j) C. Wang, X. Huang, X. Liu, et al., Chin. Chem. Lett. 31 (2020) 677-680.
-
[6]
(a) V. Quint, F. Morlet-Savary, J.F. Lohier, et al., J. Am. Chem. Soc. 138 (2016) 7436-7441;
(b) K. Luo, Y.Z. Chen, W.C. Yang, et al., Org. Lett. 18 (2016) 452-455;
(c) H.F. Qian, C.K. Li, Z.H. Zhou, et al., Org. Lett. 20 (2018) 5947-5951;
(d) H. Zhang, Z. Zhan, Y. Lin, et al., Org. Chem. Front. 5 (2018) 1416-1422;
(e) D. Liu, M.J. Jiao, Z.T. Feng, et al., Org. Lett. 20 (2018) 5700-5704;
(f) J. Yuan, W.P. To, Z.Y. Zhang, et al., Org. Lett. 20 (2018) 7816-7820;
(g) M. Singsardar, A. Dey, R. Sarkar, et al., J. Org. Chem. 83 (2018) 12694-12701;
(h) D. Liu, J.Q. Chen, X.Z. Wang, et al., Adv. Synth. Catal. 359 (2017) 2773-2777;
(i) P. Xie, J. Fan, Y. Liu, et al., Org. Lett. 20 (2018) 3341-3344.
-
[7]
(a) B. Zhang, A. Studer, Chem. Soc. Rev. 44 (2015) 3505-3521;
(b) J. Lei, J. Huang, Q. Zhu, Org. Biomol. Chem. 14 (2016) 2593-2602;
(c) B. Song, B. Xu, Chem. Soc. Rev. 46 (2017) 1103-1123.
-
[8]
(a) Y. Liu, X.L. Chen, F.L. Zeng, et al., J. Org. Chem. 83 (2018) 11727-11735;
(b) D. Li, T. Mao, J. Huang, et al., Org. Lett. 19 (2017) 3223-3226;
(c) B. Zhang, C.G. Daniliuc, A. Studer, Org. Lett. 16 (2014) 250-253;
(d) Y. Gao, J. Wu, J. Xu, et al., Asian J. Org. Chem. 3 (2014) 691-694;
(e) J.J. Cao, T.H. Zhu, Z.Y. Gu, et al., Tetrahedron 70 (2014) 6985-6990;
(f) Y. Li, G. Qiu, Q. Ding, et al., Tetrahedron 70 (2014) 4652-4656;
(g) W.C. Yang, K. Wei, X. Sun, et al., Org. Lett. 20 (2018) 3144-3147.
-
[9]
(a) K. Luo, W.C. Yang, L. Wu, Asian J. Org. Chem. 6 (2017) 350-367;
(b) B.G. Cai, J. Xuan, W.J. Xiao, Sci. Bull. 64 (2019) 337-350.
-
[10]
(a) C.X. Li, D.S. Tu, R. Yao, et al., Org. Lett. 18 (2016) 4928-4931;
(b) C.H. Wang, Y.H. Li, S.D. Yang, Org. Lett. 20 (2018) 2382-2385.
-
[11]
(a) C.G. Mortimer, G. Wells, J.P. Crochard, et al., J. Med. Chem. 49 (2006) 179-185;
(b) S. Aiello, G. Wells, E.L. Stone, et al., J. Med. Chem. 51 (2008) 5135-5139.
-
[12]
(a) T. He, L. Yu, L. Zhang, et al., Org. Lett. 13 (2011) 5016-5019;
(b) C. Li, P. Li, J. Yang, et al., Chem. Commun. 48 (2012) 4214-4216;
(c) P. Peng, L. Peng, G. Wang, et al., Org. Chem. Front. 3 (2016) 749-752;
(d) L. Li, J.J. Wang, G.W. Wang, J. Org. Chem. 81 (2016) 5433-5439.
-
[13]
(a) J. Xu, F. Zhang, S. Zhang, et al., Org. Lett. 21 (2019) 1112-1115;
(b) D.P. Hari, T. Hering, B. KoÌnig, Org. Lett. 14 (2012) 5334-5337;
(c) J. Xu, X. Yu, J. Yan, et al., Org. Lett. 19 (2017) 6292-6295;
(d) J. Yan, J. Xu, Y. Zhou, et al., Org. Chem. Front. 5 (2018) 1483-1487;
(e) Y. Liu, X.L. Chen, K. Sun, et al., Org. Lett. 21 (2019) 4019-4024;
(f) Y. Gao, P. Zhao, G. Li, et al., J. Org. Chem. 83 (2018) 13726-13733;
(g) Y. Yuan, W. Dong, X. Gao, et al., Org. Lett. 21 (2019) 469-472;
(h) T. Cai, J. Liu, H. Zhang, et al., Org. Lett. 21 (2019) 4605-4608;
(i) Z. Cao, Q. Zhu, Y.W. Lin, et al., Chin. Chem. Lett. 30 (2019) 2132-2138;
(j) F.L. Zeng, X.L. Chen, S.Q. He, et al., Org. Chem. Front. 6 (2019) 1476-1480;
(k) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262;
(l) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290;
(m) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70.
-
[14]
(a) W. Yang, S. Yang, P. Li, et al., Chem. Commun. 51 (2015) 7520-7523;
(b) W.C. Yang, P. Dai, K. Luo, et al., Adv. Synth. Catal. 359 (2017) 2390-2395;
(c) W.C. Yang, P. Dai, K. Luo, et al., Adv. Synth. Catal. 358 (2016) 3184-3190;
(d) W. Yang, T. Miao, P. Li, et al., RSC Adv. 5 (2015) 95833-95839;
(e) W.C. Yang, J.G. Feng, L. Wu, et al., Adv. Synth. Catal. 361 (2019) 1700-1709;
(f) D. Li, W.C. Yang, Tetrahedron Lett. 60 (2019) 1792-1795;
(g) X. Zhang, W. Yang, L. Wang, Org. Biomol. Chem. 11 (2013) 3649-3654.
-
[15]
(a) J. Xuan, T.T. Zeng, J.R. Chen, et al., Chem.-Eur. J. 21 (2015) 4962-4965;
(b) M.J. Bu, G.P. Lu, C. Cai, Catal. Sci. Technol. 6 (2016) 413-416.

 Login In
Login In







 DownLoad:
DownLoad: