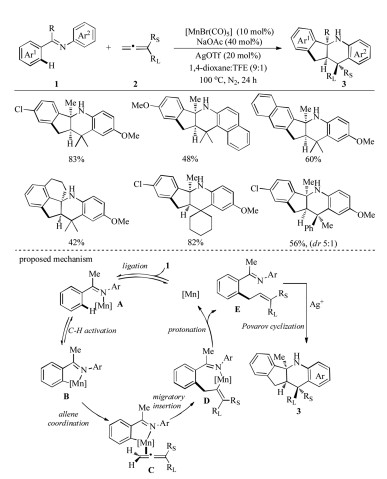
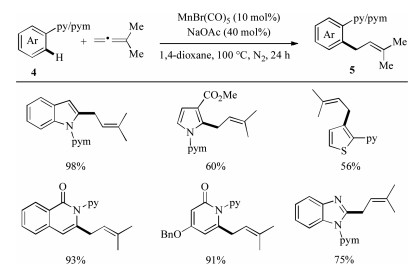
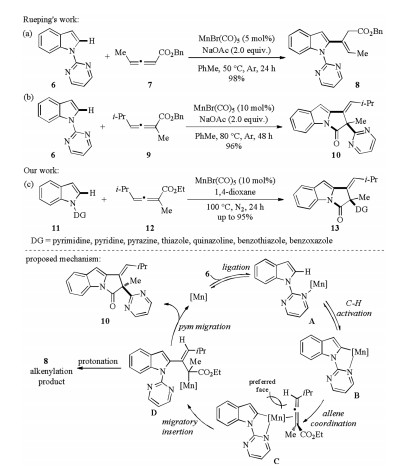
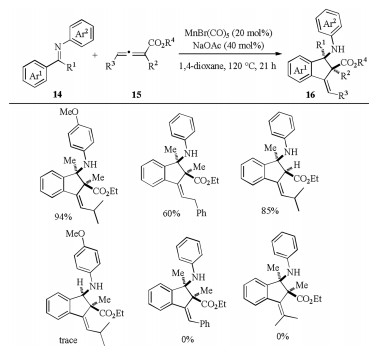
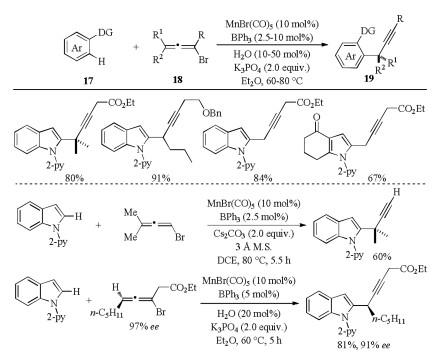
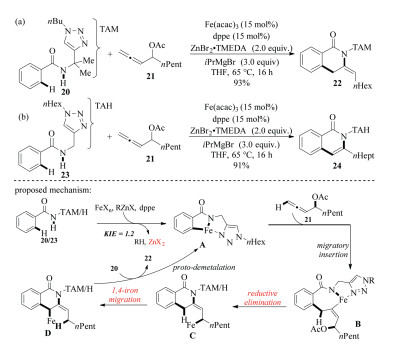
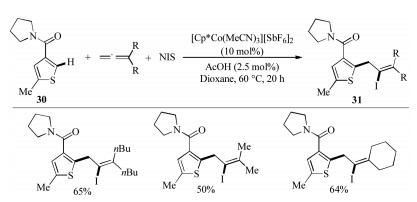
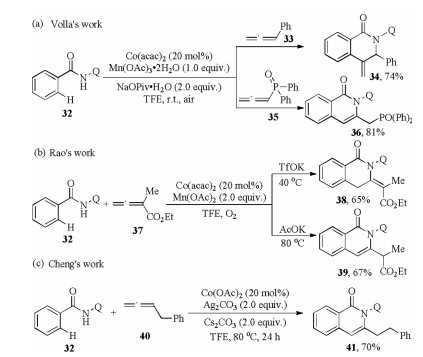
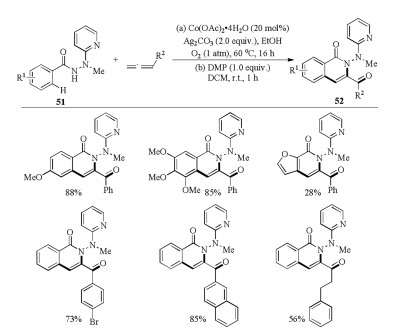
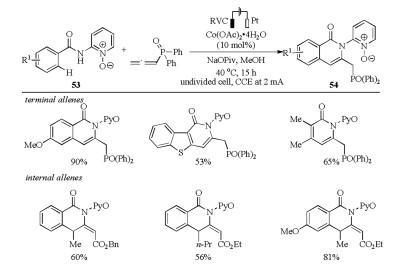
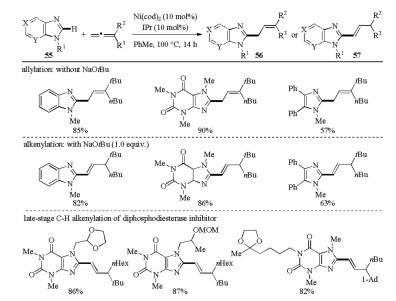
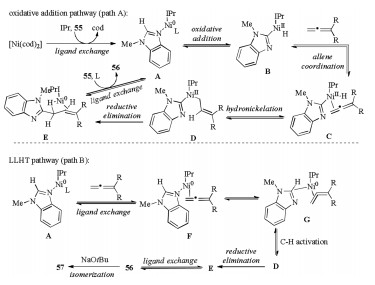
-
[1]
(a) P. Gandeepan, T. Müller, D. Zell, et al., Chem. Rev. 119 (2019) 2192-2452;
(b) Y. Hu, B. Zhou, C. Wang, Acc. Chem. Res. 51 (2018) 816-827;
(c) S. Prakash, R. Kuppusamy, C.H. Cheng, ChemCatChem 10 (2018) 683-705;
(d) Y. Xu, G. Dong, Chem. Sci. 9 (2018) 1424-1432;
(e) J. He, M. Wasa, K.S.L. Chan, Q. Shao, J.Q. Yu, Chem. Rev. 117 (2017) 8754-8786;
(f) J.R. Hummel, J.A. Boerth, J.A. Ellman, Chem. Rev. 117 (2017) 9163-9227;
(g) Y. Park, Y. Kim, S. Chang, Chem. Rev. 117 (2017) 9247-9301;
(h) J. Jiao, K. Murakami, K. Itami, ACS Catal. 6 (2016) 610-633;
(i) W. Liu, L. Ackermann, ACS Catal. 6 (2016) 3743-3752;
(j) T. Gensch, M.N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 45 (2016) 2900-2936;
(k) F. Wang, S. Yu, X. Li, Chem. Soc. Rev. 45 (2016) 6462-6477;
(l) M. Gulías, J.L. Mascareñas, Angew. Chem. Int. Ed. 55 (2016) 11000-11019;
(m) B. Ye, N. Cramer, Acc. Chem. Res. 48 (2015) 1308-1318;
(n) O. Daugulis, J. Roane, L.D. Tran, Acc. Chem. Res. 48 (2015) 1053-1064;
(o) B. Liu, F. Hu, B.F. Shi, ACS Catal. 48 (2015) 1863-1881;
(p) S.A. Girard, T. Knauber, C.J. Li, Angew. Chem. Int. Ed. 53 (2014) 74-100;
(q) G. Rouquet, N. Chatani, Angew. Chem. Int. Ed. 52 (2013) 11726-11743;
(r) X. Shang, Z.Q. Liu, Chem. Soc. Rev. 42 (2013) 3253-3260;
(s) B.J. Li, Z.J. Shi, Chem. Soc. Rev. 41 (2012) 5588-5598;
(t) L. McMurray, F. O'Hara, M.J. Gaunt, Chem. Soc. Rev. 40 (2011) 1885-1898;
(u) C.S. Yeung, V.M. Dong, Chem. Rev. 111 (2011) 1215-1292;
(v) T. Newhouse, P.S. Baran, Angew. Chem. Int. Ed. 50 (2011) 3362-3374;
(w) T.W. Lyons, M.S. Sanford, Chem. Rev. 110 (2010) 1147-1169.
-
[2]
B.M. Trost, Science 254 (1991) 1471-1477.
doi: 10.1126/science.1962206
-
[3]
P.A. Wender, B.L. Miller, Nature 460 (2009) 197-201.
doi: 10.1038/460197a
-
[4]
(a) A. Hoffmann-Röder, N. Krause, Angew. Chem. Int. Ed. 43 (2004) 1196-1216;
(b) P. Rivera-Fuentes, F. Diederich, Angew. Chem. Int. Ed. 51 (2012) 2818-2828;
(c) S. Yu, S. Ma, Angew. Chem. Int. Ed. 51 (2012) 3074-3112.
-
[5]
(a) B. Yang, Y. Qiu, J.E. Bäckvall, Acc. Chem. Res. 51 (2018) 1520-1531;
(b) J. Ye, S. Ma, Acc. Chem. Res. 47 (2014) 989-1000;
(c) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria, A. Simonneau, Chem. Rev. 111 (2011) 1954-1993;
(d) S. Ma, Chem. Rev. 105 (2005) 2829-2872;
(e) A.S.K. Hashmi, Angew. Chem. Int. Ed. 39 (2000) 3590-3593;
(f) N. Krause, A.S. Hashmi, Modern Allene Chemistry, Wiley-VCH, Weinheim, 2004.
-
[6]
Y.J. Zhang, E. Skucas, M.J. Krische, Org. Lett. 11 (2009) 4248-4250.
doi: 10.1021/ol901759t
-
[7]
(a) Z. Fang, C. Fu, S. Ma, Chem. -Eur. J. 16 (2010) 3910-3913;
(b) R. Zeng, C. Fu, S. Ma, J. Am. Chem. Soc. 134 (2012) 9597-9600;
(c) R. Zeng, S. Wu, C. Fu, S. Ma, J. Am. Chem. Soc. 135 (2013) 18284-18287.
-
[8]
S. Nakanowatari, L. Ackermann, Chem. -Eur. J. 21 (2015) 16246-16251.
doi: 10.1002/chem.201502785
-
[9]
P. Gandeepan, P. Rajamalli, C.H. Cheng, Chem. -Eur. J. 21 (2015) 9198-9203.
doi: 10.1002/chem.201501106
-
[10]
(a) H. Wang, F. Glorius, Angew. Chem. Int. Ed. 51 (2012) 7318-7322;
(b) H. Wang, B. Beiring, D.G. Yu, K.D. Collins, F. Glorius, Angew. Chem. Int. Ed. 52 (2013) 12430-12434.
-
[11]
(a) D.N. Tran, N. Cramer, Angew. Chem. Int. Ed. 49 (2010) 8181-8184;
(b) D.N. Tran, N. Cramer, Angew. Chem. Int. Ed. 52 (2013) 10630-10634;
(c) B. Ye, N. Cramer, J. Am. Chem. Soc. 135 (2013) 636-639.
-
[12]
(a) T. Lan, L. Wang, Y. Rao, Org. Lett. 19 (2017) 972-975;
(b) T. Li, C. Zhang, Y. Tan, W. Pan, Y. Rao, Org. Chem. Front. 4 (2017) 204-209;
(c) X. Yao, L. Jin, Y. Rao, Asian J. Org. Chem. 6 (2017) 825-830.
-
[13]
(a) Y. Kuninobu, P. Yu, K. Takai, Org. Lett. 12 (2010) 4274-4276;
(b) X.F. Xia, Y.Q. Wang, L.L. Zhang, et al., Chem.-Eur. J. 20 (2014) 5087-5091;
(c) T.J. Gong, W. Su, Z.J. Liu, et al., Org. Lett. 16 (2014) 330-333.
-
[14]
R. Kuppusamy, K. Muralirajan, C.H. Cheng, ACS Catal. 6 (2016) 3909-3913.
doi: 10.1021/acscatal.6b00978
-
[15]
R. Santhoshkumar, C.H. Cheng, Asian J. Org. Chem. 7 (2018) 1151-1163.
doi: 10.1002/ajoc.201800133
-
[16]
C. Wang, Synlett 24 (2013) 1606-1613.
doi: 10.1055/s-0033-1339299
-
[17]
S.Y. Chen, Q. Li, X.G. Liu, et al., ChemSusChem 10 (2017) 2360-2364.
doi: 10.1002/cssc.201700452
-
[18]
S.Y. Chen, Q. Li, H. Wang, J. Org. Chem. 82 (2017) 11173-11181.
doi: 10.1021/acs.joc.7b02220
-
[19]
C. Wang, A. Wang, M. Rueping, Angew. Chem. Int. Ed. 56 (2017) 9935-9938.
doi: 10.1002/anie.201704682
-
[20]
S.Y. Chen, X.L. Han, J.Q. Wu, et al., Angew. Chem. Int. Ed. 56 (2017) 9939-9943.
doi: 10.1002/anie.201704952
-
[21]
(a) Y. Kuninobu, Y. Nishina, T. Takeuchi, K. Takai, Angew. Chem. Int. Ed. 46 (2007) 6518-6520;
(b) B. Zhou, Y. Hu, C. Wang, Angew. Chem. Int. Ed. 54 (2015) 13659-13663;
(c) W. Liu, J. Bang, Y. Zhang, L. Ackermann, Angew. Chem. Int. Ed. 54 (2015) 14137-14140;
(d) Y.F. Liang, L. Massignan, W. Liu, L. Ackermann, Chem. -Eur. J. 22 (2016) 14856-14859;
(e) S. Sueki, Z. Wang, Y. Kuninobu, Org. Lett. 18 (2016) 304-307;
(f) B. Zhou, Y. Hu, T. Liu, C. Wang, Nat. Commun. 8 (2017) 1169-1177.
-
[22]
C. Lei, L. Peng, K. Ding, Adv. Synth. Catal. 360 (2018) 2952-2958.
doi: 10.1002/adsc.201800465
-
[23]
C. Zhu, J.L. Schwarz, S. Cembellín, S. Greβies, F. Glorius, Angew. Chem. Int. Ed. 57 (2018) 437-441.
doi: 10.1002/anie.201710835
-
[24]
(a) P. Wang, L. Deng, Chin. J. Chem. 36 (2018) 1222-1240;
(b) R. Shang, L. Ilies, E. Nakamura, Chem. Rev. 117 (2017) 9086-9139;
(c) F. Jia, Z. Li, Org. Chem. Front. 1 (2014) 194-214.
-
[25]
J. Mo, T. Müller, J.C.A. Oliveira, L. Ackermann, Angew. Chem. Int. Ed. 57 (2018) 7719-7723.
doi: 10.1002/anie.201801324
-
[26]
(a) T. Yoshino, S. Matsunaga, Adv. Synth. Catal. 359 (2017) 1245-1262;
(b) S. Wang, S.Y. Chen, Chem. Commun. 53 (2017) 3165-3180.
-
[27]
S. Nakanowatari, R. Mei, M. Feldt, L. Ackermann, ACS Catal. 7 (2017) 2511-2515.
doi: 10.1021/acscatal.7b00207
-
[28]
J.P. Wan, L. Gan, Y. Liu, Org. Biomol. Chem. 15 (2017) 9031-9043.
doi: 10.1039/C7OB02011B
-
[29]
J.A. Boerth, J.A. Ellman, Angew. Chem. Int. Ed. 56 (2017) 9976-9980.
doi: 10.1002/anie.201705817
-
[30]
N. Thrimurtulu, A. Dey, D. Maiti, C.M.R. Volla, Angew. Chem. Int. Ed. 55 (2016) 12361-12365.
doi: 10.1002/anie.201604956
-
[31]
R. Boobalan, R. Kuppusamy, R. Santhoshkumar, P. Gandeepan, C.H. Cheng, ChemCatChem 9 (2017) 273-277.
doi: 10.1002/cctc.201601190
-
[32]
N. Thrimurtulu, R. Nallagonda, C.M.R. Volla, Chem. Commun. 53 (2017) 1872-1875.
doi: 10.1039/C6CC08622E
-
[33]
R. Kuppusamy, R. Santhoshkumar, R. Boobalan, H.R. Wu, C.C. Cheng, ACS Catal. 8 (2018) 1880-1883.
doi: 10.1021/acscatal.7b04087
-
[34]
R. Boobalan, R. Santhoshkumar, C.H. Cheng, Adv. Synth. Catal. 361 (2019) 1140-1145.
doi: 10.1002/adsc.201801335
-
[35]
S. Zhai, S. Qiu, X. Chen, et al., ACS Catal. 8 (2018) 6645-6649.
doi: 10.1021/acscatal.8b01720
-
[36]
T.H. Meyer, J.C.A. Oliveira, S.C.S. Sau, N.W.J. Ang, L. Ackermann, ACS Catal. 8 (2018) 9140-9147.
doi: 10.1021/acscatal.8b03066
-
[37]
S. Nakanowatari, T. Müller, J.C.A. Oliveira, L. Ackermann, Angew. Chem. Int. Ed. 56 (2017) 15891-15895.
doi: 10.1002/anie.201709087

 Login In
Login In






 DownLoad:
DownLoad: