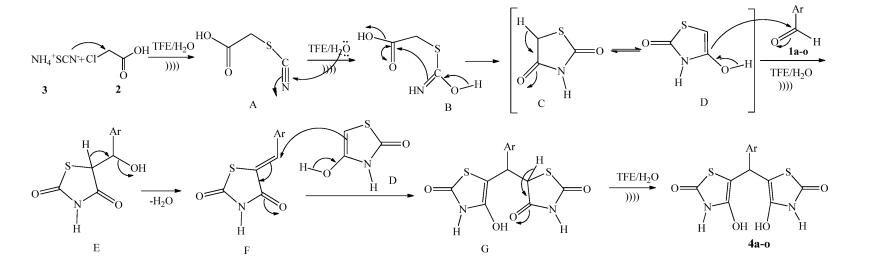
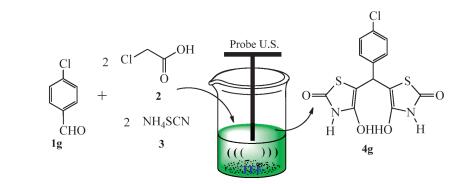
-
[1]
P. T. Anastas, J. C. Warner, Green Chemistry, 1998, Theory and Practice, Oxford University Press, Oxford, UK, 1998.
-
[2]
P. T. Anastas, T. Williamson, Green Chemistry, Frontiers in Benign Chemical Synthesis and Process, Oxford University Press, Oxford, UK, 1998.
-
[3]
Begv J.P., Crousse B., Bonnet D., Delpon -. Fluorinated alcohols:a new medium for selective and clean reaction[J]. Synlett,
2004:18-29.
-
[4]
Ratnikov M.O., Tumanov V.V., Smit W.A.. Lewis acid catalyst free electrophilic alkylation of silicon-capped π donors in 1, 11, 3, 3, 3-hexafluoro-2-propanol[J]. Angew. Chem. Int. Ed.,
2008,47:9739-9742.
doi: 10.1002/anie.v47:50
-
[5]
Wojtyk J.T.C., Kazmaier P.M., Buncel E.. Effects of metal ion complexation on the spiropyran-merocyanine interconversion:development of a thermally stable photo-switch[J]. Chem. Commun,
1998:1703-1704.
-
[6]
Gravotto G., Cintas P.. Power ultrasound in organic synthesis:moving cavitational chemistry from academia to innovative and large-scale applications[J]. Chem. Soc. Rev.,
2006,35:180-196.
doi: 10.1039/B503848K
-
[7]
Muravyova E.A., Desenko S.M., Musatov V.I.. Ultrasonic-promoted threecomponent synthesis of some biologically active 1, 2, 5, 6-tetrahydropyrimidines[J]. J. Comb. Chem.,
2007,9:797-803.
doi: 10.1021/cc700089a
-
[8]
(a) H. Pessoa-Mahana, K. C. Johann, R. H. Nadia, et al. , Solvent-free microwave synthesis of 3-(4-benzo[b]thiophene-2-carbonyl)-1-piperazinyl-1-benzo[b] thiophen-2-yl-1-propanones. New hetero bis-ligands with potential 5-HT1A serotonergic activity, Heterocycles 75(2008) 1913-1929;
(b) J. Blagg, C. Mowbray, D. C. Pryde, et al. , Non-peptide C5 a receptor antagonists: part 1, Bioorg. Med. Chem. Lett. 18(2008) 5601-5604.
-
[9]
(a) D. Simoni, R. Romagnoli, R. Baruchello, et al. , Novel A-ring and B-ring modified combretastatin A-4(CA-4) analogues endowed with interesting cytotoxic activity, J. Med. Chem. 51(2008) 6211-6215;
(b) M. A. A. Radwan, M. A. Shehab, S. M. El-Shenawy, Synthesis and biological evaluation of 5-substituted benzo[b]thiophene derivatives as antiinflammatory agents, Monatsh. Chem. 140(2009) 445-450.
-
[10]
(a) I. M. I. Fakar, M. A. A. Radwan, S. El-Batran, O. M. E. Abd El-Salam, S. M. ElShenawy, Synthesis and pharmacological evaluation of 2-substitutedbenzo[b] thiophenes as anti-inflammatory and analgesic agents, Eur. J. Med. Chem. 44(2009) 1718-1725;
(b) R. M. V. Abreu, I. C. F. R. Ferreira, M. J. R. P. Queiroz, QSAR model for predicting radical scavenging activity of di (hetero) arylamines derivatives of benzo[b] thiophenes, Eur. J. Med. Chem. 44(2009) 1952-1958.
-
[11]
Aloup J.C., Bouchaudon J., Farge D.. Synthesis and antisecretory and antiulcer activities of derivatives and analogs of 2-(2-pyridyl) tetrahydrothiophene-2-carbothioamide[J]. Med. Chem.,
1987,30:24-29.
doi: 10.1021/jm00384a004
-
[12]
Azizi N., Hasani M., Khajeh M., Edrisi M.. A straightforward and sustainable one-pot, four-component synthesis of rhodanine derivatives[J]. Tetrahedron Lett.,
2015,56:1189-1192.
doi: 10.1016/j.tetlet.2015.01.102
-
[13]
Ramesh V., Rao B.A., Sharma P.. Synthesis and biological evaluation of new rhodanine analogues bearing 2-chloroquinoline and benzo[J]. Eur. J. Med. Chem.,
2014,83:569-580.
doi: 10.1016/j.ejmech.2014.06.013
-
[14]
Singh S.J., Chauhan S.M.S.. Potassium carbonate catalyzed one pot fourcomponent synthesis of rhodanine derivatives[J]. Tetrahedron Lett.,
2013,54:2484-2488.
doi: 10.1016/j.tetlet.2013.03.004
-
[15]
(a) D. V. Selwood, K. Carter, R. J. Young, K. S. Jandu, Synthesis and biological evaluation of the L-enantiomer of 2-deoxy-5-ethyl-4-thiouridine, Bioorg. Med. Chem. Lett. 8(1996) 991-994.
-
[16]
Singh P., Katyal A., Kalra R., Chandra R.. Copper nanoparticles in an ionic liquid:an efficient catalyst for the synthesis of bis-(4-hydroxy-2-oxothiazolyl) methanes[J]. Tetrahedron Lett.,
2008,49:727-730.
doi: 10.1016/j.tetlet.2007.11.106
-
[17]
Yuasa H., Kajimoto T., Ong W.C.H.. Synthesis of iminothiasugar as a potential transition-state analog inhibitor of glycosyltransfer reactions[J]. Tetrahedron Lett.,
1994,35:8243-8246.
doi: 10.1016/0040-4039(94)88293-2
-
[18]
(a) M. Yoshikawa, T. Murakami, H. Shimada, et al. , Salacinol, potent antidiabetic principle with unique thiosugar sulfonium sulfate structure from the Ayurvedic traditional medicine Salacia reticulata in Sri Lanka and India, Tetrahedron Lett 38(1997) 8367-8370;
(b) M. Yoshikawa, T. Murakami, K. Yashiro, H. Matsuda, A potent α-glucosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic Ayurvedic medicine Salacia reticulate, Chem. Pharm. Bull. 46(1998) 1339-1340.
-
[19]
Muravyova E.A., Desenko S.M., Musatov V.I.. Ultrasonic-promoted threecomponent synthesis of some biologically active 1, 2, 5, 6-tetrahydropyrimidines[J]. J. Comb. Chem.,
2007,9:797-803.
doi: 10.1021/cc700089a

 Login In
Login In






 DownLoad:
DownLoad: