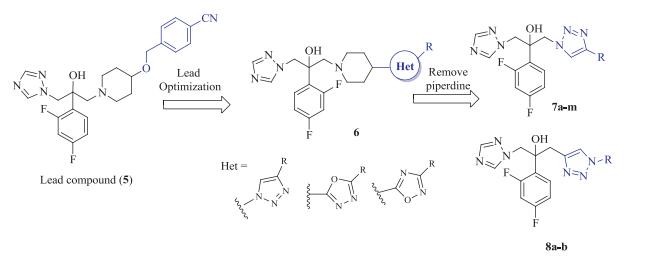
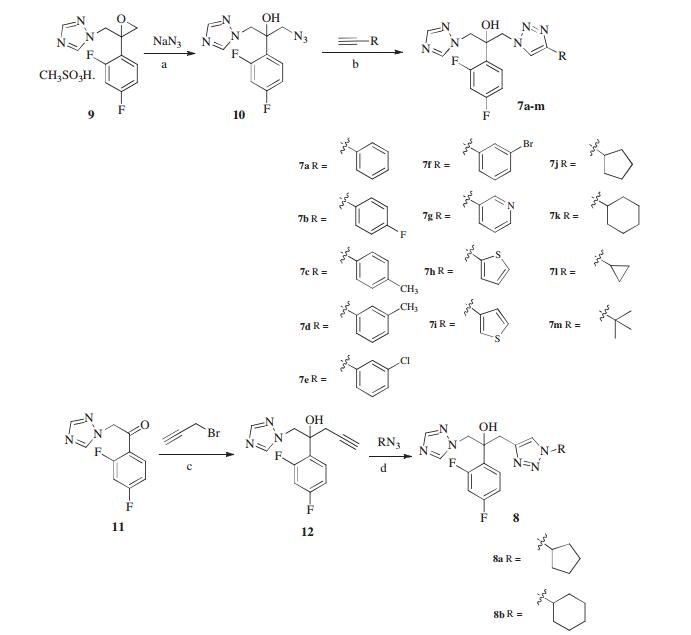
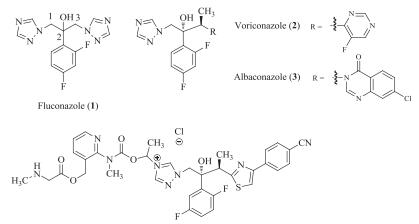
-
[1]
Enoch D.A., Ludlam H.A., Brown N.M. Invasive fungal infections:a review of epidemiology and management options[J]. J.Med.Microbiol.,
2006,55:809-818.
doi: 10.1099/jmm.0.46548-0
-
[2]
Lai C.C., Tan C.K., Huang Y.T., Shao P.L., Hsueh P.R. Current challenges in the management of invasive fungal infections[J]. J.Infect.Chemother.,
2008,14:77-85.
doi: 10.1007/s10156-007-0595-7
-
[3]
Park B.J., Wannemuehler K.A., Marston B.J.. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS[J]. AIDS,
2009,23:525-530.
doi: 10.1097/QAD.0b013e328322ffac
-
[4]
Odds F.C. Genomics, molecular targets and the discovery of antifungal drugs: genómica, dianas moleculares y el descubrimiento de fármacos antifúngicos[J]. Rev.Iberoam.Micol.,
2005,22:229-237.
doi: 10.1016/S1130-1406(05)70048-6
-
[5]
Odds F.C., Brown A.J.P., Gow N.A.R. Antifungal agents:mechanisms of action[J]. Trends Microbiol.,
2003,11:272-279.
doi: 10.1016/S0966-842X(03)00117-3
-
[6]
Ostrosky-Zeichner L., Marr K.A., Rex J.H., Cohen S.H. Amphotericin B:time for a new"gold standard"[J]. Clin.Infect.Dis.,
2003,37:415-425.
doi: 10.1086/376634
-
[7]
Fanos V., Cataldi L. Amphotericin B-induced nephrotoxicity:a review[J]. J. Chemother.,
2000,12:463-470.
doi: 10.1179/joc.2000.12.6.463
-
[8]
Pfaller M.A. Antifungal drug resistance:mechanisms, epidemiology, and consequences for treatment[J]. Am.J.Med.,
2012,125:S3-S13.
doi: 10.1016/S0002-9343(12)00756-5
-
[9]
Guillon R., Pagniez F., Rambaud C.. Design, synthesis, and biological evaluation of 1-[(biarylmethyl)methylamino] -2-(2, 4-difluorophenyl)-3-(1H-1, 2, 4-triazol-1-yl)propan-2-ols as potent antifungal agents:new insights into structure-activity relationships[J]. ChemMedChem,
2011,6:1806-1815.
doi: 10.1002/cmdc.v6.10
-
[10]
Guillon R., Pagniez F., Giraud F.. Design, synthesis, and in vitro antifungal activity of 1-[(4-substituted-benzyl)methylamino] -2-(2, 4-difluorophenyl)-3-(1H-1, 2, 4-triazol-1-yl)propan-2-ols[J]. ChemMedChem,
2011,6:816-825.
doi: 10.1002/cmdc.v6.5
-
[11]
Guillon R., Logé C., Pagniez F.. Synthesis and in vitro antifungal evaluation of 2-(2, 4-difluorophenyl)-1-[(1H-indol-3-ylmethyl)methylamino] -3-(1H-1, 2, 4-triazol-1-yl)propan-2-ols[J]. J.Enzyme Inhib.Med.Chem.,
2011,26:261-269.
doi: 10.3109/14756366.2010.503607
-
[12]
Guillon R., Giraud F., Logé C., Borgne M.Le. Design of new antifungal agents:synthesis and evaluation of 1-[(1H-indol-5-ylmethyl)amino] -2-phenyl-3-(1H-1, 2, 4-triazol-1-yl)propan-2-ols[J]. Bioorg.Med.Chem.Lett.,
2009,19:5833-5836.
doi: 10.1016/j.bmcl.2009.08.089
-
[13]
Giraud F., Guillon R., Logé C.. Synthesis and structure-activity relationships of 2-phenyl-1-[(pyridinyl-and piperidinylmethyl)amino] -3-(1H-1, 2, 4-triazol-1-yl)propan-2-ols as antifungal agents[J]. Bioorg.Med.Chem. Lett.,
2009,19:301-304.
doi: 10.1016/j.bmcl.2008.11.101
-
[14]
Pettit N.N., Carver P.L. Isavuconazole:a new option for the management of invasive fungal infections[J]. Ann.Pharmacother.,
2015,49:825-842.
doi: 10.1177/1060028015581679
-
[15]
Türel O.. Newer antifungal agents[J]. Expert.Rev.Anti Infect.Ther.,
2011,9:325-338.
doi: 10.1586/eri.10.163
-
[16]
Szpilman A.M., Carreira E.M. Probing the biology of natural products: molecular editing by diverted total synthesis[J]. Angew.Chem.Int.Ed.,
2010,49:9592-9628.
doi: 10.1002/anie.200904761
-
[17]
Sheng C.Q., Miao Z.Y., Ji H.T.. Three-dimensional model of lanosterol 14alpha-demethylase from Cryptococcus neoformans:active-site characterization and insights into azole binding[J]. Antimicrob.Agents Chemother.,
2009,53:3487-3495.
doi: 10.1128/AAC.01630-08
-
[18]
Sheng C.Q., Wang W.Y., Che X.Y.. Improved model of lanosterol 14alpha-demethylase by ligand-supported homology modeling:validation by virtual screening and azole optimization[J]. ChemMedChem,
2010,5:390-397.
doi: 10.1002/cmdc.v5:3
-
[19]
Sheng C., Zhang W., Zhang M.. Homology modeling of lanosterol 14alpha-demethylase of Candida albicans and Aspergillus fumigatus and insights into the enzyme-substrate Interactions[J]. J.Biomol.Struct.Dyn.,
2004,22:91-99.
doi: 10.1080/07391102.2004.10506984
-
[20]
Sheng C.Q., Chen S.H., Ji H.T.. Evolutionary trace analysis of CYP51 family: implication for site-directed mutagenesis and novel antifungal drug design[J]. J. Mol.Model.,
2010,16:279-284.
doi: 10.1007/s00894-009-0527-9
-
[21]
Sheng C.Q., Zhang W.N., Ji H.T.. Structure-based optimization of azole antifungal agents by CoMFA CoMSIA, and molecular docking[J]. J.Med.Chem.,
2006,49:2512-2525.
doi: 10.1021/jm051211n
-
[22]
Che X.Y., Sheng C.Q., Wang W.Y.. New azoles with potent antifungal activity:design, synthesis and molecular docking[J]. Eur.J.Med.Chem.,
2009,44:4218-4226.
doi: 10.1016/j.ejmech.2009.05.018
-
[23]
Jiang Z.G., Wang Y., Wang W.Y.. Discovery of highly potent triazole antifungal derivatives by heterocycle-benzene bioisosteric replacement[J]. Eur.J. Med.Chem.,
2013,64:16-22.
doi: 10.1016/j.ejmech.2013.04.025
-
[24]
Sheng C.Q., Che X.Y., Wang W.Y.. Design and synthesis of novel triazole antifungal derivatives by structure-based bioisosterism[J]. Eur.J.Med.Chem.,
2011,46:5276-5282.
doi: 10.1016/j.ejmech.2011.03.019
-
[25]
Sheng C.Q., Che X.Y., Wang W.Y.. Structure-based design synthesis, and antifungal activity of new triazole derivatives[J]. Chem.Biol.Drug Des.,
2011,78:309-313.
doi: 10.1111/jpp.2011.78.issue-2
-
[26]
Wang W.Y., Sheng C.Q., Che X.. Discovery of highly potent novel antifungal azoles by structure-based rational design[J]. Bioorg.Med.Chem.Lett.,
2009,19:5965-5969.
doi: 10.1016/j.bmcl.2009.07.144
-
[27]
Zhu S.P., Wang W.Y., Fang K.. Design, synthesis and antifungal activity of carbazole derivatives[J]. Chin.Chem.Lett.,
2014,25:229-233.
doi: 10.1016/j.cclet.2013.10.022
-
[28]
Wang W.Y., Wang S.Z., Liu Y.. Novel conformationally restricted triazole derivatives with potent antifungal activity[J]. Eur.J.Med.Chem.,
2010,45:6020-6026.
doi: 10.1016/j.ejmech.2010.09.070
-
[29]
Xu Y.L., Sheng C.Q., Wang W.Y.. Structure-based rational design, synthesis and antifungal activity of oxime-containing azole derivatives[J]. Bioorg.Med. Chem.Lett.,
2010,20:2942-2945.
doi: 10.1016/j.bmcl.2010.03.014
-
[30]
Wang W.Y., Wang S.Z., Dong G.Q.. Discovery of highly potent antifungal triazoles by structure-based lead fusion[J]. Med.Chem.Commun.,
2011,2:1066-1072.
doi: 10.1039/c1md00103e
-
[31]
Jiang Z.G., Gu J.L., Wang C., Wang S.. Design, synthesis and antifungalactivity of novel triazole derivatives containing substituted 1, 2, 3-triazole-piperdine side chains[J]. Eur.J.Med.Chem.,
2014,82:490-497.
doi: 10.1016/j.ejmech.2014.05.079
-
[32]
Sheng C.Q., Che X.Y., Wang W.Y.. Design and synthesis of antifungal benzoheterocyclic derivatives by scaffold hopping[J]. Eur.J.Med.Chem.,
2011,46:1706-1712.
doi: 10.1016/j.ejmech.2011.01.075
-
[33]
Wu S.C., Zhang Y.Q., He X.M.. From antidiabetic to antifungal:discovery of highly potent triazole-thiazolidinedione hybrids as novel antifungal agents[J]. ChemMedChem,
2014,9:2639-2646.
doi: 10.1002/cmdc.v9.12
-
[34]
He X.M., Jiang Y., Zhang Y.Q.. Discovery of highly potent triazoleantifungal agents with piperidine-oxadiazole side chains[J]. Med. Chem.Commun.,
2015,6:653-664.
doi: 10.1039/C4MD00505H
-
[35]
Aher N.G., Pore V.S., Mishra N.N.. Synthesis and antifungal activity of 1, 2, 3-triazole containing fluconazole analogues[J]. Bioorg.Med.Chem.Lett.,
2009,19:759-763.
doi: 10.1016/j.bmcl.2008.12.026
-
[36]
Pore V.S., Aher N.G., Kumarb M., Shukla P.K. Design and synthesis of fluconazole/bile acid conjugate using click reaction[J]. Tetrahedron,
2006,62:11178-11186.
doi: 10.1016/j.tet.2006.09.021
-
[37]
Yu S.C., Wang N., Chai X.Y.. Synthesis and antifungal activity of the novel triazole derivatives containing 1, 2, 3-triazole fragment[J]. Arch.Pharm.Res.,
2013,36:1215-1222.
doi: 10.1007/s12272-013-0063-0
-
[38]
Okoli I., Coleman J.J., Tampakakis E.. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay[J]. PLoS One,
2009,4e7025.
doi: 10.1371/journal.pone.0007025

 Login In
Login In






 DownLoad:
DownLoad: