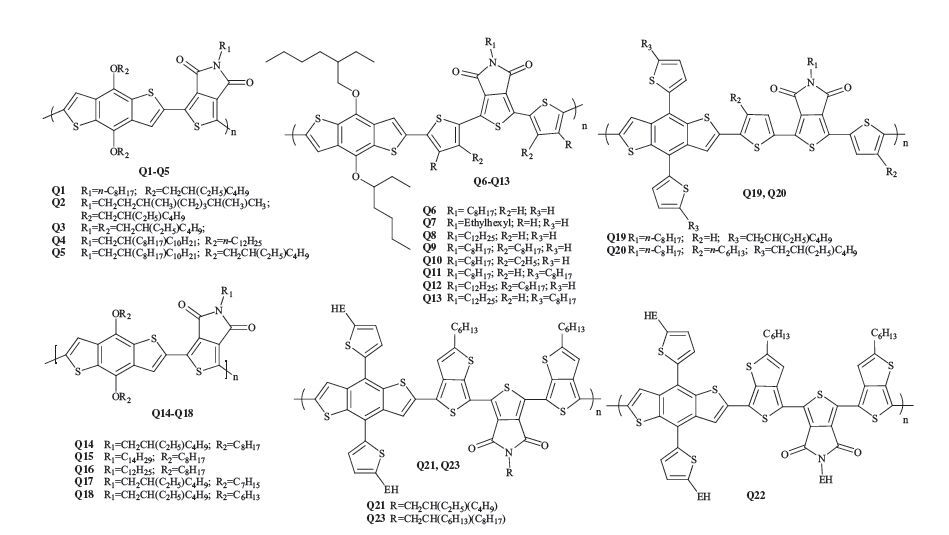
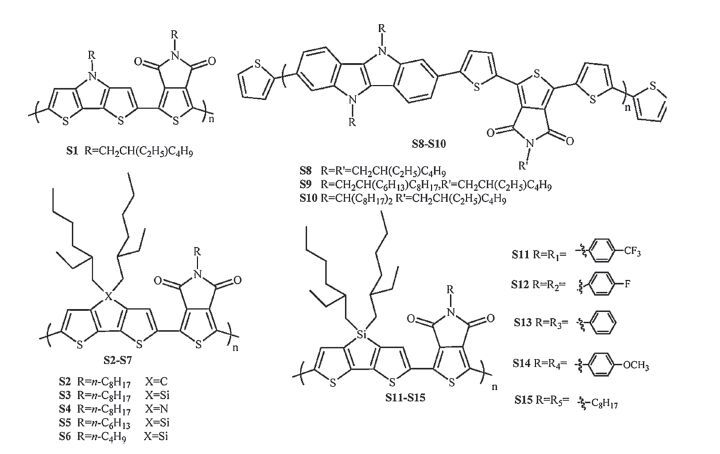
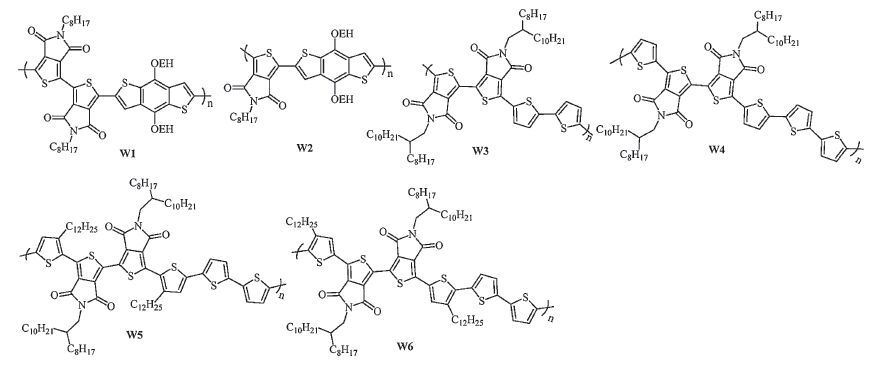
-
[1]
Z. Cai, Y. Guo, S. Yang. New donor-acceptor-donor molecules with pechmann dye as the core moiety for solution-processed good-performance organic field-effect transistors[J]. Chem. Mater.,
2013,25:471-478.
doi: 10.1021/cm303793g
-
[2]
S.S. Dharmapurikar, A. Arulkashmir, C. Das. Enhanced hole carrier transport due to increased intermolecular contacts in small molecule based field effect transistors[J]. ACS Appl. Mater. Interfaces,
2013,5:7086-7093.
doi: 10.1021/am401379a
-
[3]
S. Xu, N. Ai, J. Heng, N. Zhao. Extended isoindigo core: synthesis and applications as solution-processable n-OFET materials in ambient conditions[J]. RSC Adv.,
2015,5:8340-8344.
doi: 10.1039/C4RA14072A
-
[4]
X. Ren, S. Jiang, M. Cha. Thiophene-bridged double D-π-A dye for efficient dye-sensitized solar cell[J]. Chem. Mater.,
2012,24:3493-3499.
doi: 10.1021/cm302250y
-
[5]
X. He, B. Gao, T.C. Hauger. Donor-acceptor small molecules for organic photovoltaics: single-atom substitution (Se or S)[J]. ACS Appl. Mater. Interfaces,
2015,7:8188-8199.
doi: 10.1021/acsami.5b01063
-
[6]
A. Mishra, M.K. Fischer, P. Bauerle. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules[J]. Angew. Chem. Int. Ed.,
2009,48:2474-2499.
doi: 10.1002/anie.v48:14
-
[7]
Q. Zhang, J.M. Tour. Alternating donor/acceptor repeat units in polythiophenes intramolecular charge transfer for reducing band gaps in fully substituted conjugated polymers[J]. J. Am. Chem. Soc.,
1998,120:5356-5362.
-
[8]
A. Pron, P. Berrouard, M. Leclerc. Thieno[J]. Macromol. Chem. Phys.,
2013,214:7-16.
doi: 10.1002/macp.201200549
-
[9]
M. Yuan, M. Chiu, S. Liu. A thieno[3, 4-c]pyrrole-4, 6-dione-based donor-acceptor polymer exhibiting high crystallinity for photovoltaic applications[J]. Macromolecule,
2010,43:6936-6938.
doi: 10.1021/ma101523a
-
[10]
T. Umeyama, M. Oodoi, O. Yoshikawa. Synthesis and photovoltaic properties of thiophene-imide-fused thiophene alternating copolymers with different alkyl side chains[J]. J. Mater. Chem.,
2011,21:12454-12461.
doi: 10.1039/c1jm11531f
-
[11]
Q. Wu, M. Wang, X. Qiao. Thieno[J]. Macromolecules,
2013,46:3887-3894.
doi: 10.1021/ma400544s
-
[12]
X. Guo, R.P. Ortiz, Y. Zheng. Thieno[J]. J. Am. Chem. Soc.,
2011,133:13685-13697.
doi: 10.1021/ja205398u
-
[13]
N. Zhou, A.S. Dudnik, T.I.N.G. Li. All-polymer solar cell performance optimized via systematic molecular weight tuning of both donor and acceptor polymers[J]. J. Am. Chem. Soc.,
2016,138:1240-1251.
doi: 10.1021/jacs.5b10735
-
[14]
X. Wang, C. Gao, K. Wang. Synthesis and electronic energy-level regulation of imide-fused poly(thienoylene vinylene) derivatives[J]. Polym. Chem.,
2013,51:4975-4982.
doi: 10.1002/pola.v51.23
-
[15]
Y.R. Cheon, Y.J. Kim, J. Ha. TPD-based copolymers with strong interchain aggregation and high hole mobility for efficient bulk heterojunction solar cells[J]. Macromolecule,
2014,47:8570-8577.
doi: 10.1021/ma501888z
-
[16]
J.W. Jung, T.P. Russell, W.H. Jo. Highly crystalline low band gap polymer based on thieno[J]. ACS Appl. Mater. Interfaces,
2015,7:13666-13674.
doi: 10.1021/acsami.5b03446
-
[17]
W. Qing, Z. Liu, S. Yang. Modulating carrier transfer ability-linker effect on thieno[J]. RSC Adv.,
2015,5:55619-55624.
doi: 10.1039/C5RA08723F
-
[18]
Y. Zou, A. Najari, P. Berrouard. A thieno[3, 4-c]pyrrole-4, 6-dione-based copolymer for efficient solar cells[J]. J. Am. Chem. So,
2010,132:5330-5331.
doi: 10.1021/ja101888b
-
[19]
Y. Zhang, S.K. Hau, H. Yip. Efficient polymer solar cells based on the copolymers of benzodithiophene and thienopyrroledione[J]. Chem. Mater.,
2010,22:2696-2698.
doi: 10.1021/cm100417z
-
[20]
C. Piliego, T.W. Holcombe, J.D. Douglas. Synthetic control of structural order in N-alkylthieno[J]. J. Am. Chem. Soc.,
2010,132:7595-7597.
doi: 10.1021/ja103275u
-
[21]
G. Zhang, Y. Fu, Q. Zhang. Benzo[J]. Chem. Commum.,
2010,46:4997-4999.
doi: 10.1039/c0cc00098a
-
[22]
A. Najari, S. Beaupre, P. Berrouard. Synthesis and new characterization of thieno[J]. Adv. Funct. Mater.,
2011,21:718-728.
doi: 10.1002/adfm.201001771
-
[23]
B.R. Aich, J. Lu, S. Beaupre. Control of the active layer nanomorphology by using co-additives towards high-performance bulk heterojunction solar cells[J]. Org. Electron.,
2012,13:1736-1741.
doi: 10.1016/j.orgel.2012.05.001
-
[24]
B. Cabanetos, A.E. Labban, J.A. Bartelt. Linear side chains in benzo[J]. J. Am. Chem. Soc.,
2013,135:4656-4659.
doi: 10.1021/ja400365b
-
[25]
K. Lu, J. Fang, H. Yan. A facile strategy to enhance absorption coefficient and photovoltaic performance of two-dimensional benzo[1, 2-b:4, 5-b'] dithiophene and thieno[3, 4-c]pyrrole-4, 6-dione polymers via subtle chemical structure variations[J]. Org. Electro,
2013,14:2652-2661.
doi: 10.1016/j.orgel.2013.07.006
-
[26]
C. Zhang, H. Li, J. Wang. Low-bandgap thieno[J]. J. Mater. Chem. A,
2015,3:11194-11198.
doi: 10.1039/C5TA02376A
-
[27]
X. Hu, M. Shi, L. Zuo. Synthesis, characterization, and photovoltaic property of a low band gap polymer alternating dithienopyrrole and thienopyrroledione units[J]. Polymer,
2011,52:2559-2564.
doi: 10.1016/j.polymer.2011.03.057
-
[28]
Y. Zhang, J. Zou, H. Yip. Conjugated polymers based on C, Si and Nbridged dithiophene and thienopyrroledione units: synthesis, field-effect transistors and bulk heterojunction polymer solar cells[J]. J. Mater. Chem.,
2011,21:3895-3902.
doi: 10.1039/c0jm03927f
-
[29]
T. Chu, J. Lu, S. Beaupre. Bulk heterojunction solar cells using thieno[J]. J. Am. Chem. Soc.,
2011,133:4250-4253.
doi: 10.1021/ja200314m
-
[30]
T. Chu, J. Lu, S. Beaupre. Effects of the molecular weight and the side-chain length on the photovoltaic performance of dithienosilole/thienopyrrolodione copolymers[J]. Adv. Funct. Mater.,
2012,22:2345-2351.
doi: 10.1002/adfm.v22.11
-
[31]
C.M. Amb, S. Chen, K.R. Graham. Dithienogermole as a fused electron donor in bulk heterojunction solar cells[J]. J. Am. Chem. Soc.,
2011,133:10062-10065.
doi: 10.1021/ja204056m
-
[32]
Z.R. Owczarczyk, W.A. Braunecker, A. Garcia. 5, 10-Dihydroindolo[3, 2-b]indole-based copolymers with alternating donor and acceptor moieties for organic photovoltaics[J]. Macromolecules,
2013,46:1350-1360.
doi: 10.1021/ma301987p
-
[33]
T. Ikai, T. Kudo, M. Nagaki. Fine tuning of frontier orbital energy levels in dithieno[J]. Polymers,
2014,55:2139-2145.
doi: 10.1016/j.polymer.2014.03.021
-
[34]
P. Berrouard, F. Grenier, J. Pouliot. Synthesis and characterization of 5-octylthieno[J]. Org. Lett.,
2011,13:38-41.
doi: 10.1021/ol1027514
-
[35]
X. Qiao, Q. Wu, H. Wu. High performance thin film transistors based on bithieno[J]. Polym. Chem.,
2016,7:807-815.
doi: 10.1039/C5PY01995H

 Login In
Login In






 DownLoad:
DownLoad: