Citation:
Huo Jing-Qian, Ma Liu-Yong, Zhang Zhe, Fan Zhi-Jin, Zhang Jin-Lin, Tetyana V. Beryozkina, Vasiliy A. Bakulev. Synthesis and biological activity of novel N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amides derivatives[J]. Chinese Chemical Letters,
;2016, 27(9): 1547-1550.
doi:
10.1016/j.cclet.2016.06.019

-
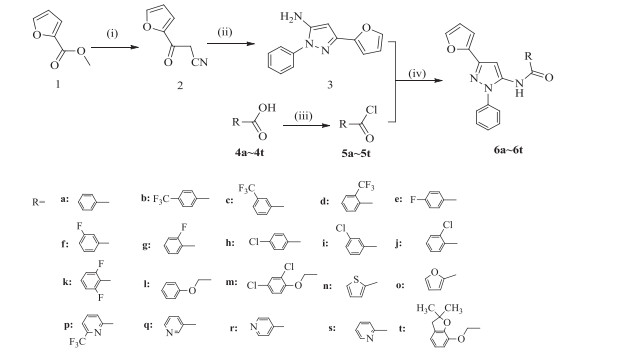
A series of novel N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amides derivatives were designed and synthesized. Their structures were confirmed by 1H NMR, 13C NMR and HRMS. All title compounds were evaluated for their herbicidal and antifungal activities. Preliminary bioassay results indicated that the title compounds showed good to moderate herbicidal activity at 1000 mg/L. Compound 6q presented the best activity against Digitaria sanguinalis (L) Scop., Amaranthus retroflexus L. and Arabidopsis thaliana with an inhibition degree of five. Compound 6d also showed an inhibition degree of five against D. sanguinalis. In addition, at 50 mg/L, most compounds exhibited good in vitro antifungal activity against Sclerotinia sclerotiorum, with compound 6c showing over 90% antifungal activity against S. sclerotiorum and Pellicularia sasakii.
-
Keywords:
- Amides derivative,
- Pyrazole,
- Furan,
- Synthesis,
- Herbicidal activity,
- Fungicidal activity
-

-
-
[1]
Umina P.A., Weeks A.R., Roberts J.. The current status of pesticide resistance in Australian populations of the redlegged earth mite (Halotydeus destructor)[J]. Pest Manag. Sci., 2012,68:889-896. doi: 10.1002/ps.v68.6
-
[2]
Norsworthy J.K., Ward S.M., Shaw D.R.. Reducing the risks of herbicide resistance:best management practices and recommendations[J]. Weed Sci., 2012,60:31-62. doi: 10.1614/WS-D-11-00155.1
-
[3]
T. Van Leeuwen, W. Dermauw, M. Grbic, L. Tirry, R. Feyereisen, Spider mite control and resistance management:does a genome help? Pest Manag. Sci. 69(2013) 156-159.
-
[4]
H. Sierotzki, G. Scalliet, A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides, Phytopathology 103(2013) 880-887.
-
[5]
Consortium R.E.X.. Heterogeneity of selection and the evolution of resistance[J]. Trends Ecol. Evol., 2013,28:110-118. doi: 10.1016/j.tree.2012.09.001
-
[6]
J.W.H. Li, J.C. Vederas, Drug discovery and natural products:end of an era or an endless frontier? Science 325(2009) 161-165.
-
[7]
Duke S.O., Dayan F.E., Rimando A.M.. Invited paper:chemicals from nature for weed management[J]. Weed Sci., 2002,50:138-151. doi: 10.1614/0043-1745(2002)050[0138:IPCFNF]2.0.CO;2
-
[8]
Matsuura B.S., Keylor M.H., Li B.. A scalable biomimetic synthesis of resveratrol dimers and systematic evaluation of their antioxidant activities[J]. Angew. Chem. Int. Ed. Engl., 2015,54:3754-3757. doi: 10.1002/anie.201409773
-
[9]
Wang T., Wu M.B., Chen Z.J.. Fragment-based drug discovery and molecular docking in drug design[J]. Curr. Pharm. Biotechnol., 2015,16:11-25. doi: 10.2174/1389201015666141122204532
-
[10]
Gengenbacher M., Dick T.. Antibacterial drug discovery:doing it right[J]. Chem. Biol., 2015,22:5-6. doi: 10.1016/j.chembiol.2014.12.005
-
[11]
Lindert S., Li M.X., Sykes B.D., McCammon J.A.. Computer-aided drug discovery approach finds calcium sensitizer of cardiac troponin[J]. Chem. Biol. Drug Des., 2015,85:99-106. doi: 10.1111/cbdd.12381
-
[12]
Zhao B., Huo J.Q., Xing J.H.. Homologous modeling of transketolase AtTKL1 and its combination with a-terthienyl in Arabidopsis thaliana[J]. Chem. J. Chin. Univ., 2015,36:682-686.
-
[13]
Murphy D.J., Walker D.A.. The properties of transketolase from photosynthetic tissue[J]. Planta, 1982,155:316-320. doi: 10.1007/BF00429458
-
[14]
Villafranca J.J., Axelrod B.. Heptulose synthesis from nonphosphorylated aldoses and ketoses by spinach transketolase[J]. J. Biol. Chem., 1971,246:3126-3131.
-
[15]
Henkes S., Sonnewald U., Badur R., Flachmann R., Stitt M.. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism[J]. Plant Cell, 2001,13:535-551. doi: 10.1105/tpc.13.3.535
-
[16]
J. Zheng, Y.Q. Liu, J.Y. Lv, Synthesis of methyl-furoate, Fine Chem. Intermed. 37(2007) 41-42, 45.
-
[17]
Ma L.C., Yuan L.W., Xu C.Z.. An efficient synthesis of 2-aminothiophenes via the gewald reaction catalyzed by an N-methylpiperazine-functionalized polyacrylonitrile fiber[J]. Synthesis, 2013,45:45-52.
-
[18]
Zhang J.L., Zhang L.H., Liu Y.C.. The herbicidal activity of mutant isolates from Botrytis cinerea[J]. Agric. Sci. China, 2006,5:622-628. doi: 10.1016/S1671-2927(06)60102-8
-
[19]
Zhang L.H., Kang Z.H., Xu J., Xu W.C., Zhang J.L.. Isolation and structural indentification of herbicidal toxin fractions produced by Pythium aphanidermatum[J]. Agric. Sci. China, 2010,9:995-1000. doi: 10.1016/S1671-2927(09)60182-6
-
[20]
Fan S.L., Zhang B., Gao L.Y.. Synthesis, antifungal activity and structureactivity relationship of 2-methoxycarbonyl/ethoxycarbonyl-4-fluorophenyl-1, 5-benzothiazepines[J]. Chem. J. Chin. Univ., 2014,35:2574-2583.
-
[21]
Kondratieva M.L., Pepeleva A.V., Belskaia N.P.. A new synthetic method for the 2H-[J]. Tetrahedron, 2007,63:3042-3048. doi: 10.1016/j.tet.2007.01.059
-
[22]
Efimov I., Bakulev V., Beliaev N.. Reactions of b-azolylenamines with sulfonyl azides as an approach to N-unsubstituted 1, 2, 3-triazoles and ethene-1, 2-diamines[J]. Eur. J. Org. Chem., 2014,2014:3684-3689. doi: 10.1002/ejoc.201402130
-
[23]
T.V. Beryozkina, I.V. Efimov, W.M.F. Fabian, et al., Reactivity of 1, 2, 3-triazoles towards sulfonyl chlorides. a novel approach to 1-and 2-sulfonyl-4-azolyl-1, 2, 3-triazoles, Tetrahedron 71(2015) 6189-6195.
-
[24]
Su W.N., Lin T.P., Cheng K.M.. An efficient one-pot synthesis of N-(1, 3-diphenyl-1H-pyrazol-5-yl) amides[J]. J. Heterocycl. Chem., 2010,47:831-837. doi: 10.1002/jhet.343
-
[1]
-

-
-
[1]
Tingting Du , Siyu Lu , Zongnan Zhu , Mei Zhu , Yan Zhang , Jian Zhang , Jixiang Chen . Pyrazole derivatives: Recent advances in discovery and development of pesticides. Chinese Chemical Letters, 2025, 36(9): 110912-. doi: 10.1016/j.cclet.2025.110912
-
[2]
Yu Mao , Yilin Liu , Xiaochen Wang , Shengyang Ni , Yi Pan , Yi Wang . Acylfluorination of enynes via phosphine and silver catalysis. Chinese Chemical Letters, 2024, 35(8): 109443-. doi: 10.1016/j.cclet.2023.109443
-
[3]
Yulong Shi , Fenbei Chen , Mengyuan Wu , Xin Zhang , Runze Meng , Kun Wang , Yan Wang , Yuheng Mei , Qionglu Duan , Yinghong Li , Rongmei Gao , Yuhuan Li , Hongbin Deng , Jiandong Jiang , Yanxiang Wang , Danqing Song . Chemical construction and anti-HCoV-OC43 evaluation of novel 10,12-disubstituted aloperine derivatives as dual cofactor inhibitors of TMPRSS2 and SR-B1. Chinese Chemical Letters, 2024, 35(5): 108792-. doi: 10.1016/j.cclet.2023.108792
-
[4]
Huiju Cao , Lei Shi . sp1-Hybridized linear and cyclic carbon chain. Chinese Chemical Letters, 2025, 36(4): 110466-. doi: 10.1016/j.cclet.2024.110466
-
[5]
Chao LIU , Jiang WU , Zhaolei JIN . Synthesis, crystal structures, and antibacterial activities of two zinc(Ⅱ) complexes bearing 5-phenyl-1H-pyrazole group. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1986-1994. doi: 10.11862/CJIC.20240153
-
[6]
Anqiu LIU , Long LIN , Dezhi ZHANG , Junyu LEI , Kefeng WANG , Wei ZHANG , Junpeng ZHUANG , Haijun HAO . Synthesis, structures, and catalytic activity of aluminum and zinc complexes chelated by 2-((2,6-dimethylphenyl)amino)ethanolate. Chinese Journal of Inorganic Chemistry, 2024, 40(4): 791-798. doi: 10.11862/CJIC.20230424
-
[7]
Guoping Yang , Zhoufu Lin , Xize Zhang , Jiawei Cao , Xuejiao Chen , Yufeng Liu , Xiaoling Lin , Ke Li . Assembly of Y(Ⅲ)-containing antimonotungstates induced by malic acid with catalytic activity for the synthesis of imidazoles. Chinese Chemical Letters, 2024, 35(12): 110274-. doi: 10.1016/j.cclet.2024.110274
-
[8]
Yao HUANG , Yingshu WU , Zhichun BAO , Yue HUANG , Shangfeng TANG , Ruixue LIU , Yancheng LIU , Hong LIANG . Copper complexes of anthrahydrazone bearing pyridyl side chain: Synthesis, crystal structure, anticancer activity, and DNA binding. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 213-224. doi: 10.11862/CJIC.20240359
-
[9]
Wen-Rui Li , Ru-Bing Wang , Huiqiang Wang , Jin-Yao Yong , Yu-Huan Li , Shi-Shan Yu , Shuang-Gang Ma . Ring-reorganization strategy for asymmetric synthesis of sesquiterpenoid illihenin A and its antiviral activity evaluation. Chinese Chemical Letters, 2025, 36(11): 110945-. doi: 10.1016/j.cclet.2025.110945
-
[10]
Maitri Bhattacharjee , Rekha Boruah Smriti , R. N. Dutta Purkayastha , Waldemar Maniukiewicz , Shubhamoy Chowdhury , Debasish Maiti , Tamanna Akhtar . Synthesis, structural characterization, bio-activity, and density functional theory calculation on Cu(Ⅱ) complexes with hydrazone-based Schiff base ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1409-1422. doi: 10.11862/CJIC.20240007
-
[11]
Xiaomeng Hu , Jie Yu , Lijie Sun , Linfeng Zhang , Wei Zhou , Dongpeng Yan , Xinrui Wang . Synthesis of an AVB@ZnTi-LDH composite with synergistically enhance UV blocking activity and high stability for potential application in sunscreen formulations. Chinese Chemical Letters, 2024, 35(11): 109466-. doi: 10.1016/j.cclet.2023.109466
-
[12]
Bofei JIA , Zhihao LIU , Zongyuan GAO , Shuai ZHOU , Mengxiang WU , Qian ZHANG , Xiamei ZHANG , Shuzhong CHEN , Xiaohan YANG , Yahong LI . Cu(Ⅱ) and Cu(Ⅰ) complexes based on derivatives of imidazo[1,5-a]pyridine: Synthesis, structures, in situ metal-ligand reactions, and catalytic activity. Chinese Journal of Inorganic Chemistry, 2025, 41(5): 1020-1036. doi: 10.11862/CJIC.20240317
-
[13]
Zhi-Peng Bao , Hefei Yang , Ru-Han A , Yuanrui Wang , Xiao-Feng Wu . Carbonylative five-component synthesis of amides and esters with α-quaternary carbon center. Chinese Chemical Letters, 2025, 36(11): 111150-. doi: 10.1016/j.cclet.2025.111150
-
[14]
Xinyi Zhang , Kai Ren , Yanning Liu , Zhenyi Gu , Zhixiong Huang , Shuohang Zheng , Xiaotong Wang , Jinzhi Guo , Igor V. Zatovsky , Junming Cao , Xinglong Wu . Progress on Entropy Production Engineering for Electrochemical Catalysis. Acta Physico-Chimica Sinica, 2024, 40(7): 2307057-0. doi: 10.3866/PKU.WHXB202307057
-
[15]
Lei Feng , Ze-Min Zhu , Ying Yang , Zongbin He , Jiafeng Zou , Man-Bo Li , Yan Zhao , Zhikun Wu . Long-Pursued Structure of Au23(S-Adm)16 and the Unexpected Doping Effects. Acta Physico-Chimica Sinica, 2024, 40(5): 2305029-0. doi: 10.3866/PKU.WHXB202305029
-
[16]
Ying Chen , Ronghua Yan , Weiyan Yin . Research Progress on the Synthesis of Metal Single-Atom Catalysts and Their Applications in Electrocatalytic Hydrogen Evolution Reactions. University Chemistry, 2025, 40(9): 344-353. doi: 10.12461/PKU.DXHX202503066
-
[17]
Jiaming Xu , Yu Xiang , Weisheng Lin , Zhiwei Miao . Research Progress in the Synthesis of Cyclic Organic Compounds Using Bimetallic Relay Catalytic Strategies. University Chemistry, 2024, 39(3): 239-257. doi: 10.3866/PKU.DXHX202309093
-
[18]
Huashan Huang , Jingze Chen , Luyun Zhang , Hong Yan , Siqi Li , Fen-Er Chen . Oscillatory flow reactor facilitates fast photochemical Wolff rearrangement toward synthesis of α-substituted amides in flow. Chinese Chemical Letters, 2025, 36(2): 109992-. doi: 10.1016/j.cclet.2024.109992
-
[19]
Guangyao Wang , Zhitong Xu , Ye Qi , Yueguang Fang , Guiling Ning , Junwei Ye . Electrospun nanofibrous membranes with antimicrobial activity for air filtration. Chinese Chemical Letters, 2024, 35(10): 109503-. doi: 10.1016/j.cclet.2024.109503
-
[20]
Ting Wang , Xin Yu , Yaqiang Xie . Unlocking stability: Preserving activity of biomimetic catalysts with covalent organic framework cladding. Chinese Chemical Letters, 2024, 35(6): 109320-. doi: 10.1016/j.cclet.2023.109320
-
[1]
Metrics
- PDF Downloads(1)
- Abstract views(1126)
- HTML views(22)

 Login In
Login In




 DownLoad:
DownLoad: