Citation:
Sun Shangzheng, Wang Xing, Cheng Taijin, Xu Hui, Dai Huixiong. Cu(II)-Mediated β-C—H Alkynylation of Acrylamides with Terminal Alkynes[J]. Chinese Journal of Organic Chemistry,
;2020, 40(10): 3371-3379.
doi:
10.6023/cjoc202005064

-
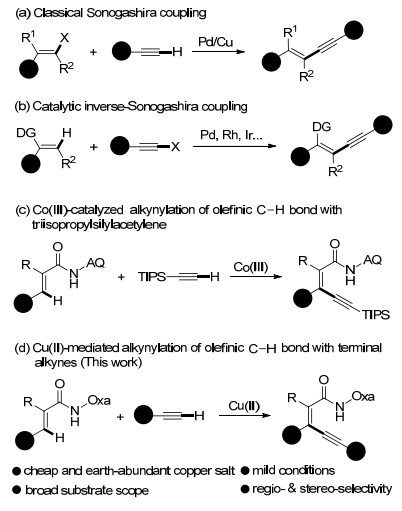
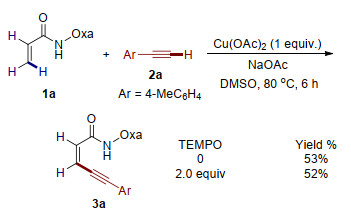
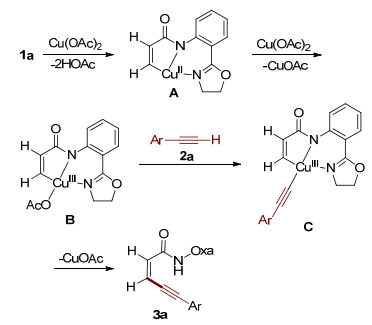
Cu(Ⅱ)-mediated β-C-H alkynylation of acrylamides with terminal alkynes is described by employing amide-oxazoline bidentate auxiliary, forming the conjugated 1, 3-enynes. This protocol is characterized by its mild conditions, broad substarate scope and excellent regio- and stereo-selectivity.
-
Keywords:
- β-C-H alkynylation,
- copper,
- 1, 3-enynes,
- stereo-selectivity
-

-
-
[1]
For selected examples, see:
(a) Nussbaumer, P.; Leitner, I.; Mraz, K.; Stütz, A. J. Med. Chem. 1995, 38, 1831.
(b) Saito, S.; Yamamoto, Y. Chem. Rev. 2000, 100, 2901.
(c) Rudi, A.; Schleyer, M.; Kashman, Y. J. Nat. Prod. 2000, 63, 1434. (d) Liu, Y.; Nishiura, M.; Wang, Y.; Hou, Z. J. Am. Chem. Soc. 2006, 128, 5592. -
[2]
Daly, J. W.; Karle, I.; Myers, C. W.; Tokuyama, T.; Waters, J. A.; Witkop, B. Proc. Natl. Acad. Sci U. S. A. 1971, 68, 1870.
-
[3]
Iverson, S. L.; Uetrecht, J. P. Chem. Res. Toxicol. 2001, 14, 175. doi: 10.1021/tx0002029
-
[4]
Zein, N.; Sinha, A. M.; McGahren, W. J. Ellestad, G. A. Science 1988, 240, 11988.
-
[5]
Selected examples:
(a) Miki, K.; Nishino, F.; Ohe, K.; Uemura, S. J. Am. Chem. Soc. 2002, 124, 5260.
(b) Kawasaki, T.; Saito, S.; Yamamoto, Y. J. Org. Chem. 2002, 67, 2653.
(c) Lee, S.; Lee, T.; Lee, Y. M.; Kim, D.; Kim, S. Angew. Chem., Int. Ed. 2007, 46, 8422.
(d) Zhang, W.; Xu, H.; Xu, H. Tang, W. J. Am. Chem. Soc. 2009, 131, 3832.
(e) Nishimura, A.; Ohashi, M.; Ogoshi, S. J. Am. Chem. Soc. 2012, 134, 15692.
(f) Ma, K.; Miao, Y.; Gao, X.; Chao, J.; Zhang, X.; Qin, X.-M. Chin. Chem. Lett. 2017, 28, 1035. -
[6]
Zhou, Y.; Zhang, Y.; Wang, J. Org. Biomol. Chem. 2016, 14, 6638. doi: 10.1039/C6OB00944A
-
[7]
(a) Sonogashira, K. J. Organomet. Chem. 2002, 653, 46.
(b) Negishi, E.; Anastasia, L. Chem. Rev. 2003, 103, 1979.
(c) Plenio, H. Angew. Chem., Int. Ed. 2008, 47, 6954.
(d) Chinchilla, R.; Najera, C. Chem. Soc. Rev. 2011, 40, 5084. -
[8]
Reviews for transition metal catalyzed C-H activation, see:
(a) Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074.
(b) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
(c) Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242.
(d) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(e) Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. Chem. Rev. 2019, 119, 2192.
(f) Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120, 1788.
(g) Wang, Q.; Gu, Q.; You, S. L. Acta Chim. Sinica 2019, 77, 690(in Chinese).
(王强, 顾庆, 游书力, 化学学报, 2019, 77, 690.)
(h) Guan, H.; Chen, L.; Liu, L. Acta Chim. Sinica 2018, 76, 440(in Chinese).
(关弘浩, 陈磊, 刘磊, 化学学报, 2018, 76, 440.)
(i) Li, X.; Liang, G.; Shi, Z. Chin. J. Chem. 2020, 38, 929. -
[9]
Examples for olefinic C-H alkynylation with alkynyl halides, see:
(a) Collins, K. D.; Lied, F.; Glorius, F. Chem. Commun. 2014, 50, 4459.
(b) Feng, C.; Feng, D.; Loh, T.-P. Chem. Commun. 2014, 50, 9865.
(c) Feng, C.; Feng, D.; Luo, Y.; Loh, T.-P. Org. Lett. 2014, 16, 5956.
(d) Xu, Y.-H.; Zhang, Q.-C.; He, T.; Meng, F.-F.; Loh, T.-P. Adv. Synth. Catal. 2014, 356, 1539.
(e) Finkbeiner, P.; Kloeckner, U.; Nachtsheim, B. J. Angew. Chem., Int. Ed. 2015, 54, 4949.
(f) Tan, E.; Quino-nero, O.; Elena de Orbe, M.; Echavarren, A. M. ACS Catal. 2018, 8, 2166. -
[10]
For C-H alkynylation of arenes with terminal alkynes:
(a) Wei, Y.; Zhao, H.; Kan, J.; Su, W.; Hong, M. J. Am. Chem. Soc. 2010, 132, 2522.
(b) de Haro, T.; Nevado, C. J. Am. Chem. Soc. 2010, 132, 1512.
(c) Jie, X.; Shang, Y.; Hu, P.; Su, W. Angew. Chem., Int. Ed. 2013, 52, 3630
(d) Zhou, J.; Shi, J.; Qi, Z.; Li, X.; Xu, H. E. Yi, W. ACS Catal. 2015, 5, 6999.
(e) Liu, Y.-J.; Liu, Y.-H.; Yin, X.-S.; Gu, W.-J.; Shi, B.-F. Chem.- Eur. J. 2015, 21, 205.
(f) Tian, C.; Dhawa, U.; Scheremetjew, A.; Ackermann, L. ACS Catal. 2019, 9, 7690. -
[11]
(a) Zhao, T.; Qin, D.; Han, W.; Yang, S.; Feng, B.; Gao, G.; You, J. Chem. Commun. 2019, 55, 6118.
(b) Hadi, V.; Yoo, K. S.; Jeong, M.; K. Jung, W. Tetrahedron Lett. 2009, 50, 2370.
(c) Shao, Y.-L.; Zhang, X.-H.; Han, J.-S.; Zhong, P. Org. Lett. 2012, 14, 5242. -
[12]
Select reviews for Cu-catalyzed C-H functionalization, see:
(a) Liu, J.; Chen, G.; Tan, Z. Adv. Synth. Catal. 2016, 358, 1174.
(b) Rao, W.-H.; Shi, B.-F. Org. Chem. Front. 2016, 3, 1028.
(c) Shang, M.; Sun, S.-Z.; Wang, M.; Wang, H.-Li.; Dai, H.-X. Synthesis 2016, 48, 4381. -
[13]
(a) Shang, M.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 3354.
(b) Shang, M.; Sun, S.-Z.; Wang, H.-Li.; Laforteza, B. N.; Dai, H.-X.; Yu, J.-Q. Angew. Chem., Int. Ed. 2014, 53, 10439.
(c) Shang, M.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. Org. Lett. 2014, 16, 5666.
(d) Wang, H.-L.; Shang, M.; Sun, S.-Z.; Zhou, Z.-L.; Laforteza, B. N.; Dai, H.-X.; Yu, J.-Q. Org. Lett. 2015, 17, 1228.
(e) Sun, S.-Z; Shang, M.; Wang, H.-L.; Lin, H.-X.; Dai, H.-X.; Yu, J.-Q. J. Org. Chem. 2015, 80, 8843.
(f) Shang, M.; Shao, Q.; Sun, S.-Z.; Chen, Y.-Q.; Dai, H.-X.; Yu, J.-Q. Chem. Sci. 2017, 8, 1469.
(g) Xu, L.; Wang, X.; Ma, B.; Yin, M.-X.; Lin, H.-X.; Dai, H.-X.; Yu, J.-Q. Chem. Sci. 2018, 9, 5160.
(h) Sun, S.-Z.; Xu, H.; Dai, H.-X. Chin. Chem. Lett. 2019, 30, 969.
(i) Sun, S.-Z.; Shang, M.; Xu, H.; Cheng, T.-J.; Li, M.-H.; Dai, H.-X. Chem. Commun. 2020, 56, 1444. -
[14]
(a) Shang, M.; Wang, H.-L.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 11590.
(b) Shang, M.; Wang, M.-M.; Saint-Denis, T. G.; Li, M.-H.; Dai, H.-X.; Yu, J.-Q. Angew. Chem., Int. Ed. 2017, 56, 5317. -
[15]
(a) Suess, A. M.; Ertem, M. Z.; Cramer, C. J.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 9797.
(b) Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2013, 52, 4457.
-
[1]
-

-
-
[1]
Qi Li , Zi-Lu Wang , Yun-He Xu . Copper-catalyzed 1,4-silylcyanation of 1,3-enynes: A silyl radical-initiated approach for synthesis of difunctionalized allenes. Chinese Chemical Letters, 2025, 36(3): 109991-. doi: 10.1016/j.cclet.2024.109991
-
[2]
Liangfeng Yang , Liang Zeng , Yanping Zhu , Qiuan Wang , Jinheng Li . Copper-catalyzed photoredox 1,4-amidocyanation of 1,3-enynes with N-amidopyridin-1-ium salts and TMSCN: Facile access to α-amido allenyl nitriles. Chinese Chemical Letters, 2024, 35(11): 109685-. doi: 10.1016/j.cclet.2024.109685
-
[3]
Wujun Jian , Mong-Feng Chiou , Yajun Li , Hongli Bao , Song Yang . Cu-catalyzed regioselective diborylation of 1,3-enynes for the efficient synthesis of 1,4-diborylated allenes. Chinese Chemical Letters, 2024, 35(5): 108980-. doi: 10.1016/j.cclet.2023.108980
-
[4]
He Yao , Wenhao Ji , Yi Feng , Chunbo Qian , Chengguang Yue , Yue Wang , Shouying Huang , Mei-Yan Wang , Xinbin Ma . Copper-catalyzed and biphosphine ligand controlled 3,4-boracarboxylation of 1,3-dienes with carbon dioxide. Chinese Chemical Letters, 2025, 36(4): 110076-. doi: 10.1016/j.cclet.2024.110076
-
[5]
Lei Wan , Yizhou Tong , Xi Lu , Yao Fu . Cobalt-catalyzed reductive alkynylation to construct C(sp)-C(sp3) and C(sp)-C(sp2) bonds. Chinese Chemical Letters, 2024, 35(7): 109283-. doi: 10.1016/j.cclet.2023.109283
-
[6]
Jialin Huang , Liying Fu , Zhanyong Tang , Xiaoqiang Ma , Xingda Zhao , Depeng Zhao . Cross-coupling of trifluoromethylarenes with alkynes C(sp)-H bonds and azoles C(sp2)-H bonds via photoredox/copper dual catalysis. Chinese Chemical Letters, 2025, 36(7): 110505-. doi: 10.1016/j.cclet.2024.110505
-
[7]
Wei-Bin Li , Xiao-Chao Huang , Pei Liu , Jie Kong , Guo-Ping Yang . Recent advances in directing group assisted transition metal catalyzed para-selective C-H functionalization. Chinese Chemical Letters, 2025, 36(6): 110543-. doi: 10.1016/j.cclet.2024.110543
-
[8]
Zhirong Yang , Shan Wang , Ming Jiang , Gengchen Li , Long Li , Fangzhi Peng , Zhihui Shao . One stone three birds: Ni-catalyzed asymmetric allenylic substitution of allenic ethers, hydroalkylation of 1,3-enynes and double alkylation of enynyl ethers. Chinese Chemical Letters, 2024, 35(8): 109518-. doi: 10.1016/j.cclet.2024.109518
-
[9]
Shulei Hu , Yu Zhang , Xiong Xie , Luhan Li , Kaixian Chen , Hong Liu , Jiang Wang . Rh(Ⅲ)-catalyzed late-stage C-H alkenylation and macrolactamization for the synthesis of cyclic peptides with unique Trp(C7)-alkene crosslinks. Chinese Chemical Letters, 2024, 35(8): 109408-. doi: 10.1016/j.cclet.2023.109408
-
[10]
Xinghao Cai , Chen Ma , Ying Kang , Yuqiang Ren , Xue Meng , Wei Lu , Shiming Fan , Shouxin Liu . Nickel-catalyzed C(sp2)–H alkynylation of free α-substituted benzylamines using a transient directing group. Chinese Chemical Letters, 2025, 36(10): 110901-. doi: 10.1016/j.cclet.2025.110901
-
[11]
Guang Xu , Cuiju Zhu , Xiang Li , Kexin Zhu , Hao Xu . Copper-catalyzed asymmetric [4+1] annulation of yne–allylic esters with pyrazolones. Chinese Chemical Letters, 2025, 36(4): 110114-. doi: 10.1016/j.cclet.2024.110114
-
[12]
Pengfei Zhang , Qingxue Ma , Zhiwei Jiang , Xiaohua Xu , Zhong Jin . Transition-metal-catalyzed remote meta-C—H alkylation and alkynylation of aryl sulfonic acids enabled by an indolyl template. Chinese Chemical Letters, 2024, 35(8): 109361-. doi: 10.1016/j.cclet.2023.109361
-
[13]
Zhaodong WANG . In situ synthesis, crystal structure, and magnetic characterization of a trinuclear copper complex based on a multi-substituted imidazo[1,5-a]pyrazine scaffold. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 597-604. doi: 10.11862/CJIC.20240268
-
[14]
Yan-Bo Li , Yi Li , Liang Yin . Copper(Ⅰ)-catalyzed diastereodivergent construction of vicinal P-chiral and C-chiral centers facilitated by dual "soft-soft" interaction. Chinese Chemical Letters, 2024, 35(7): 109294-. doi: 10.1016/j.cclet.2023.109294
-
[15]
Tong Li , Leping Pan , Yan Zhang , Jihu Su , Kai Li , Kuiliang Li , Hu Chen , Qi Sun , Zhiyong Wang . Electrochemical construction of 2,5-diaryloxazoles via N–H and C(sp3)-H functionalization. Chinese Chemical Letters, 2024, 35(4): 108897-. doi: 10.1016/j.cclet.2023.108897
-
[16]
Yanfen PENG , Xinyue WANG , Tianbao LIU , Xiaoshuo WU , Yujing WEI . Syntheses and luminescence of four Cd(Ⅱ)/Zn(Ⅱ) complexes constructed by 1,3‐bis(4H‐1,2,4‐triazole)benzene. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1416-1426. doi: 10.11862/CJIC.20250018
-
[17]
Shaonan Tian , Yu Zhang , Qing Zeng , Junyu Zhong , Hui Liu , Lin Xu , Jun Yang . Core-shell gold-copper nanoparticles: Evolution of copper shells on gold cores at different gold/copper precursor ratios. Chinese Journal of Structural Chemistry, 2023, 42(11): 100160-100160. doi: 10.1016/j.cjsc.2023.100160
-
[18]
Ke-Ai Zhou , Lian Huang , Xing-Ping Fu , Li-Ling Zhang , Yu-Ling Wang , Qing-Yan Liu . Fluorinated metal-organic framework for methane purification from a ternary CH4/C2H6/C3H8 mixture. Chinese Journal of Structural Chemistry, 2023, 42(11): 100172-100172. doi: 10.1016/j.cjsc.2023.100172
-
[19]
Haoran Shi , Jiaxin Wang , Yuqin Zhu , Hongyang Li , Guodong Ju , Lanlan Zhang , Chao Wang . Highly selective α-C(sp3)-H arylation of alkenyl amides via nickel chain-walking catalysis. Chinese Chemical Letters, 2024, 35(7): 109333-. doi: 10.1016/j.cclet.2023.109333
-
[20]
Yujia Shi , Yan Qiao , Pengfei Xie , Miaomiao Tian , Xingwei Li , Junbiao Chang , Bingxian Liu . Rhodium-catalyzed enantioselective in situ C(sp3)−H heteroarylation by a desymmetrization approach. Chinese Chemical Letters, 2024, 35(10): 109544-. doi: 10.1016/j.cclet.2024.109544
-
[1]
Metrics
- PDF Downloads(11)
- Abstract views(1399)
- HTML views(142)

 Login In
Login In




 DownLoad:
DownLoad: