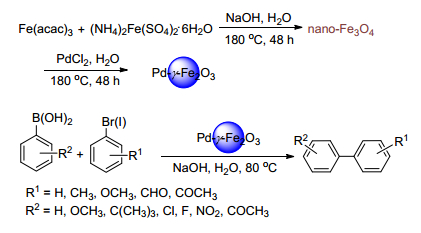
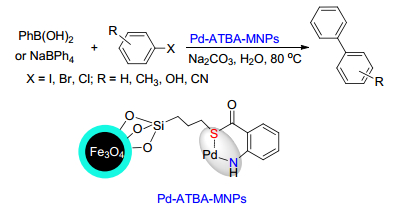
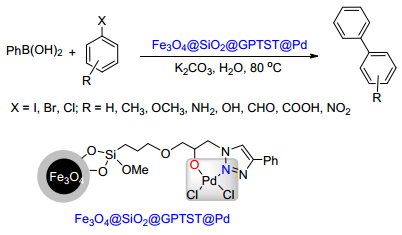
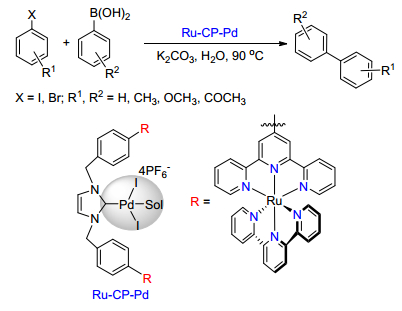
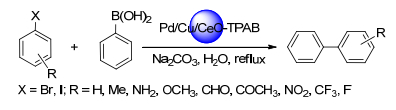
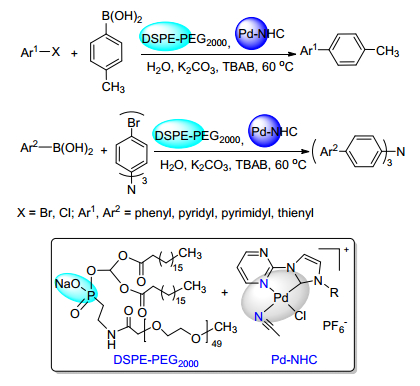
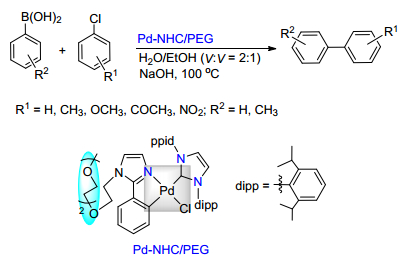
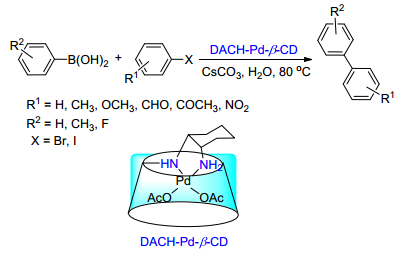
Citation:
Xu Peng, Duan Xinhong. Recent Progress in the Suzuki-Miyaura Cross-Coupling Reactions in Water[J]. Chinese Journal of Organic Chemistry,
;2019, 39(12): 3315-3327.
doi:
10.6023/cjoc201908020

-
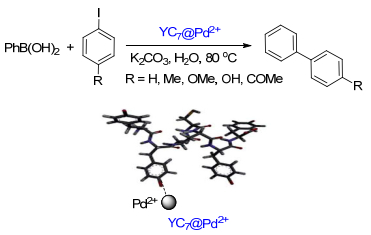
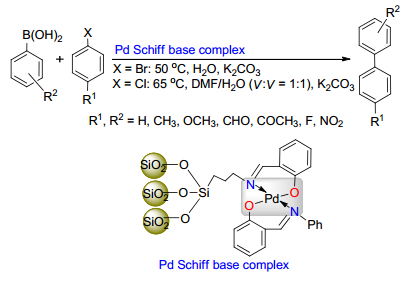
Transition-metal-catalyzed Suzuki-Miyaura cross-coupling is one of the most powerful transformations for C-C biaryl bond formation at present. With the increasing demand for green chemistry, water as solvent for the Suzuki-Miyaura cross-coupling reactions has been of widespread interest. The literature in recent years on the Suzuki-Miyaura cross-coupling reactions by heterogeneous or homogeneous catalysis in water is reviewed, and their perspectives for further developments are also presented.
-

-
-
[1]
(a) Hirner, J. J.; Shi, Y.; Blum, S. A. Accounts Chem. Res. 2011, 44, 603.
(b) Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A.-M.; Garg, N. K.; Percec, V. Chem. Rev. 2011, 111, 1346.
(c) Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417.
(d) Molnár, Á. Chem. Rev. 2011, 111, 2251.
(e) Han, F.-S. Chem. Soc. Rev. 2013, 42, 5270.
(f) Valente, C.; Calimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G. Angew. Chem. Int. Ed. 2012, 51, 3314.
(g) Knappke, C. E. I.; Grupe, S.; Gartner, D.; Corpet, M.; Gosmini, C.; Wangelin, A. J. Chem.-Eur. J. 2014, 20, 6828.
(g) Knappke, C. E. I.; Grupe, S.; Gartner, D.; Corpet, M.; Gosmini, C.; Wangelin, A. J. Chem.-Eur. J. 2014, 20, 6828.
(h) Pan, C.; Liu, M.; Duan, X. Chin. J. Org. Chem. 2015, 35, 472(in Chinese).
(潘春娇, 刘敏, 段新红, 有机化学, 2015, 35, 472.) -
[2]
Miyaura, N.; Yanagi, T.; Suzuki, A. Synth Commun. 1981, 513.
-
[3]
[(a) Shaughnessy, K. H.; Booth, R. S. Org. Lett. 2001, 3, 2757.
(b) Anderson, K. W.; Buchwald, S. L. Angew. Chem., Int. Ed. 2005, 44, 6173.
(c) Suzuki, A. Angew. Chem., Int. Ed. 2011, 50, 6723.
(d) Ge, S.; Hartwig, J. F. Angew. Chem., Int. Ed. 2012, 51, 12837.
(e) Mondal, M.; Bora, U. Green Chem. 2012, 14, 1873.
(f) Ramgren, S. D.; Hie, L.; Ye, Y.; Garg, N. K. Org. Lett. 2013, 15, 3950.
(g) Molander, G. A.; Argintaru, O. A. Org. Lett. 2014, 16, 1904. -
[4]
[(a) Ennis, D. S.; McManus, J.; Wood-Kaczmar, W.; Richardson, J.; Smith, G. E.; Carstairs, A. Org. Process Res. Dev. 1999, 3, 248.
(b) Zhou, S.-L.; Xu, L.-W.; Xia, C.-G.; Li, J.-W.; Li, F.-W. Chin. J. Org. Chem. 2004, 24, 1501(in Chinese).
(周少林, 徐利文, 夏春谷, 李经纬, 李福伟, 有机化学, 2004, 24, 1501.)
(c) Jiang, H.; Zhang, M.; Zhang, L.; Chen Y.; Zhu, N.; Song, L.; Deng, H. Chin. J. Org. Chem. 2017, 37, 2399(in Chinese).
(蒋海芳, 张敏, 张丽, 陈雅丽, 朱宁, 宋力平, 邓红梅, 有机化学, 2017, 37, 2399.) -
[5]
(a) Engberts, J. B. F. N.; Blandamer, M. J. Chem. Commun. 2001, 18, 1701.
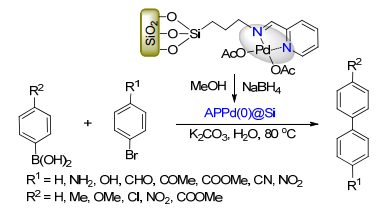
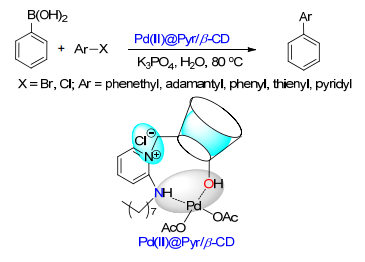
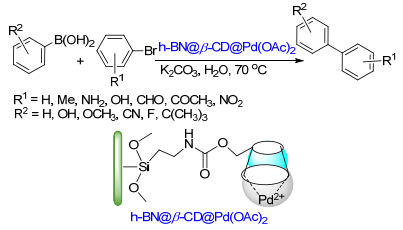
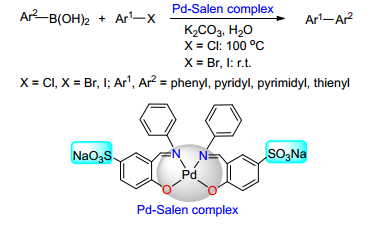
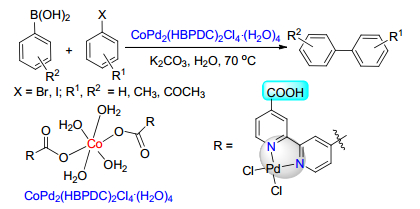
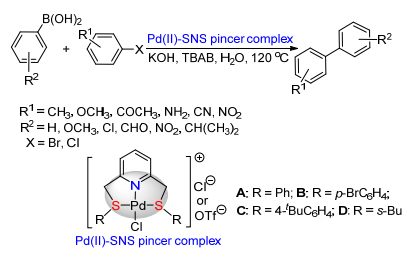
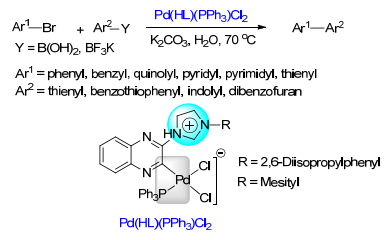
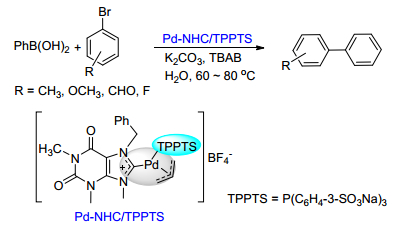
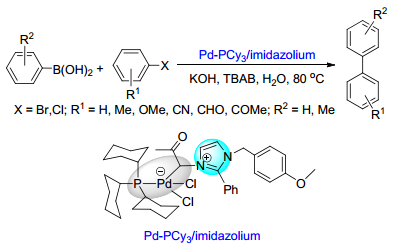
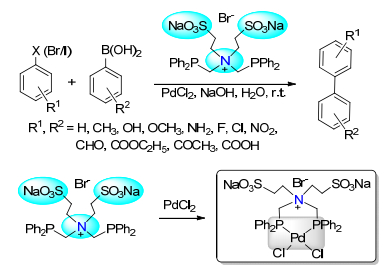
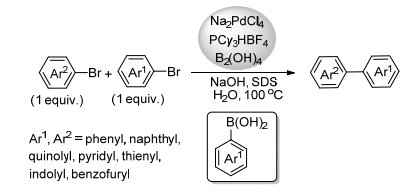
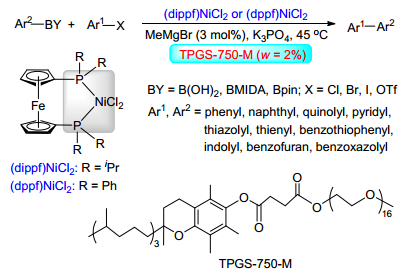
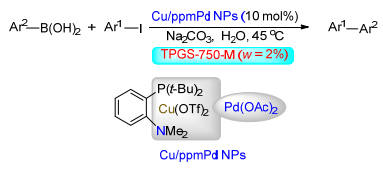
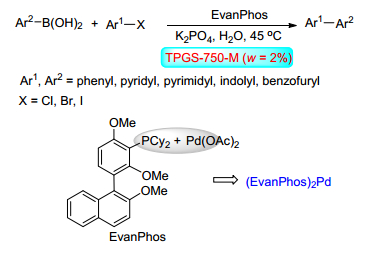
(b) Sinou, D. Adv. Synth. Catal. 2002, 344, 221.
(c) Klijn, J. E.; Engberts, J. B. F. N. Nature 2005, 435, 746.
(d) Li, C. J. Chem. Rev. 2005, 105, 3095.
(e) Shaughnessy, K. H.; DeVasher, R. B. Curr. Org. Chem. 2005, 9, 585.
(f) Li, C. J., Chen, L. Chem. Soc. Rev. 2006, 35, 68.
(g) Zhang, J.; Yin, H.; Han, S. Chin. J. Org. Chem. 2012, 32, 1429(in Chinese).
(张敬先, 殷慧清, 韩世清, 有机化学, 2012, 32, 1429.) -
[6]
Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. ChemSusChem 2010, 3, 502. doi: 10.1002/cssc.200900221
-
[7]
(a) Maluenda, I.; Navarro, O. Molecules 2015, 20, 7528.
(b) Chatterjee, A.; Ward, T. R. Catal. Lett. 2016, 146, 820. -
[8]
Li, X.; Xu, H.; Zhou, J.; Yan, G.; Zhang, L.; Zhuo, S. Chin. J. Org. Chem. 2018, 38, 1917(in Chinese).
-
[9]
(a) Joucla, L.; Batail, N.; Djakovitch, L. Adv. Synth. Catal. 2010, 352, 2929.
(b) Moncea, O.; Poinsot, D.; Fokin, A. A.; Schreiner, P. R.; Hierso, J.-C. ChemCatChem 2018, 10, 2915.
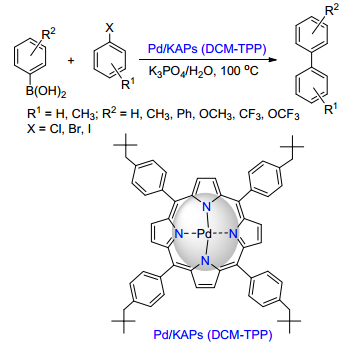
(c) Ge, C.; Sang, X.; Yao, W.; Zhang, L.; Wang, D. Green Chem. 2018, 20, 1805.
(d) Xu, Z.; Yu, X.; Sang, X.; Wang, D. Green Chem. 2018, 20, 2571.
(e) Ye, D.; Huang, R.; Zhu, H.; Zou, L.-H.; Wang, D. Org. Chem. Front. 2019, 6, 62.
(f) Ye, D.; Pan, L.; Zhu, H.; Jin, L.; Miao, H.; Wang, D. Mater. Chem. Front. 2019, 3, 216.
(g) Qiu, Y.; Zhang, Y.; Jin, L.; Pan, L.; Du, G.; Ye, D.; Wang, D. Org. Chem. Front. 2019, 6, 3420.
(h) Hu, W.; Zhang, Y.; Zhu, H.; Ye, D.; Wang, D. Green Chem. 2019, 21, 5345.
(i) Yang, Q.; Zhang, Y.; Zeng, W.; Duan, Z.-C.; Sang, X.; Wang, D. Green Chem. 2019, 21, 5683. -
[10]
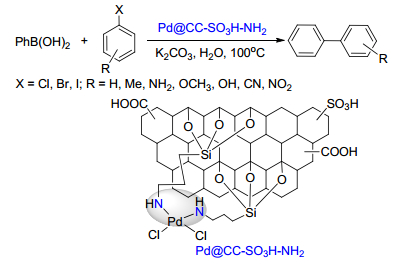
Gogoi, N.; Bora, U.; Borah, G.; Gogoi P. K. Appl. Organomet. Chem. 2016, e3686.
-
[11]
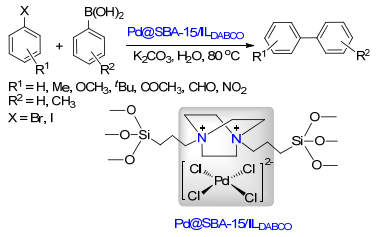
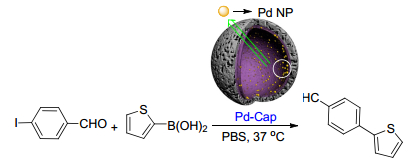
Shabbir, S.; Lee, S.; Lim, M.; Lee, H.; Ko, H.; Lee, Y.; Rhee, H. J. Organomet. Chem. 2017, 846, 296. doi: 10.1016/j.jorganchem.2017.07.003
-
[12]
Chen, J.; Zhang, J.; Zhu, D.; Li, T. Appl. Organomet. Chem. 2018, 32, e3996. doi: 10.1002/aoc.3996
-
[13]
Rathod, P. V.; Jadhav, V. H. Tetrahedron Lett. 2017, 58, 1006. doi: 10.1016/j.tetlet.2017.01.093
-
[14]
Rostamnia, S.; Doustkhah, E.; Zeynizadeh, B. Microporous Mesoporous Mater. 2016, 222, 87. doi: 10.1016/j.micromeso.2015.09.045
-
[15]
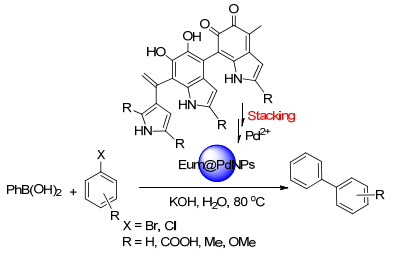
Destito, P.; Sousa-Castillo, A.; Couceiro, J. R.; López, F.; Correa-Duarte, M. A.; Mascareñas, J. L. Chem. Sci. 2019, 10, 2598 doi: 10.1039/C8SC04390F
-
[16]
Kim, Y.-O.; You, J. M.; Jang, H.-S.; Choi, S. K.; Jung, B. Y.; Kang, O.; Kim, J. W.; Lee, Y.-S. Tetrahedron Lett. 2017, 58, 2149. doi: 10.1016/j.tetlet.2017.04.062
-
[17]
Holzer, C.; Dupé, A.; Peschel, L. M.; Belaj, F.; Mösch-Zanetti, N. C. Eur. J. Inorg. Chem. 2018, 568.
-
[18]
Mandegani, Z.; Asadi, M.; Asadi, Z. Appl. Organomet. Chem. 2016, 30, 657. doi: 10.1002/aoc.3486
-
[19]
Paul, D.; Rudra, S.; Rahman, P.; Khatua, S.; Pradhan, M.; Chatterjee, P. N. J. Organomet. Chem. 2018, 871, 96. doi: 10.1016/j.jorganchem.2018.06.016
-
[20]
Ghorbani-Choghamarani, A.; Tahmasbi, B.; Moradi P. Appl. Organomet. Chem. 2016, 30, 422. doi: 10.1002/aoc.3449
-
[21]
Dadras, A.; Naimi-Jamal, M. R.; Moghaddam, F. M.; Ayati, S. E. Appl. Organomet. Chem. 2018, 32, e3993. doi: 10.1002/aoc.3993
-
[22]
(a) Ni, C.; Shen, A.; Cao, Y.; Ye, X. Chin. J. Org. Chem. 2014, 34, 278(in Chinese).
(倪晨, 沈安, 曹育才, 叶晓峰, 有机化学, 2014, 34, 278.)
(b) Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Chem. Rev. 2018, 118, 9843. -
[23]
Mondal, M.; Joji, J.; Choudhury, J. J. Chem. Sci. 2018, 130, 83. doi: 10.1007/s12039-018-1487-3
-
[24]
Mpungose, P. P.; Sehloko, N. I.; Maguire, G. E.; Friedrich, H. B. New J. Chem. 2017, 41, 13560. doi: 10.1039/C7NJ02759A
-
[25]
(a) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.
(b) Wang, D.; Yu, X.; Ge, B.; Miao, H.; Ding, Y. Chin. J. Org. Chem. 2015, 35, 676(in Chinese).
(王大伟, 余晓丽, 葛冰洋, 苗红艳, 丁玉强, 有机化学, 2015, 35, 676.)
(c) Guo, N.; Zhu, S. Chin. J. Org. Chem. 2015, 35, 1383(in Chinese).
(郭娜, 朱守非, 有机化学, 2015, 35, 1383.)
(d) Chen, S.; Yang, W.; Yao, Y.; Yang, X.; Deng, Y.; Yang, D. Chin. J. Org. Chem. 2018, 38, 2534(in Chinese).
(陈姝琪, 杨文, 姚永祺, 杨新, 邓颖颍, 杨定乔, 有机化学, 2018, 38, 2534.)
(e) Hu, X.; Yang, B.; Yao, W.; Wang, D. Chin. J. Org. Chem. 2018, 38, 3296(in Chinese).
(胡昕宇, 杨伯斌, 姚玮, 王大伟, 有机化学, 2018, 38, 3296.)
(f) Liu, N.; Chao, F.; Liu, M.-G.; Huang, N.-Y.; Zou, K.; Wang, L. J. Org. Chem. 2019, 84, 2366.
(g) Liu, M.-G.; Liu, N.; Xu, W.-H.; Wang, L. Tetrahedron 2019, 75, 2748. -
[26]
(a) Jamwal, N.; Gupta, M.; Paul, S. Green Chem. 2008, 10, 999.
(b) McLaughlin, M. P.; McCormick, T. M.; Eisenberg, R.; Holland, P. L. Chem. Commun. 2011, 47, 7989.
(c) Lipshutz, B. H.; Isley, N. A.; Moser, R.; Ghorai, S.; Leuser, H.; Taft, B. R. Adv. Synth. Catal. 2012, 354, 3175.
(d) Debnath, K.; Pathak, S.; Pramanik, A. Tetrahedron Lett. 2013, 54, 4110.
(e) Yuan, Z.-F.; Zhao, W.-N.; Liu, Z.-P.; Xu, B.-Q. J. Catal. 2017, 353, 37. -
[27]
(a) Chandrasekhar, S.; Narsihmulu, C.; Shameem Sultana, S.; Ramakrishna Reddy, N. Org. Lett. 2002, 4, 4399.
(b) Feu, K. S.; de la Torre, A. F.; Silva, S.; F de Moraes Junior, M. A.; Corrêa, A. G.; Paixão, M. W. Green Chem. 2014, 16, 3169.
(c) Tiwari, A. R.; Bhanage, B. M. Green Chem. 2016, 18, 144.
(d) Xiao, L.; Dai, F.; Li, Z.; Jing, X.; Kong, J.; Liu, G. Chin. J. Org. Chem. 2019, 39, 648(in Chinese).
(肖立伟, 戴富才, 李政, 景学敏, 孔洁, 刘光仙, 有机化学, 2019, 39, 648.) -
[28]
Chen, C.; Zheng, Q.; Ni, S.; Wang, H. New J. Chem. 2018, 42, 4624. doi: 10.1039/C7NJ04836J
-
[29]
Schroeter, F.; Soellner, J.; Strassner, T. Organometallics 2018, 37, 4267. doi: 10.1021/acs.organomet.8b00607
-
[30]
(a) Shen, H.; Ji, H. Chin. J. Org. Chem. 2011, 31, 791(in Chinese).
(沈海民, 纪红兵, 有机化学, 2011, 31, 791.)
(b) Kaboudin, B.; Abedi, Y.; Yokomatsu, T. Org. Biomol. Chem. 2012, 10, 4543.
(c) Yang, Z.; Zhang, X.; Yao, X.; Fang, Y.; Chen, H.; Ji, H. Tetrahedron 2016, 72, 1773. -
[31]
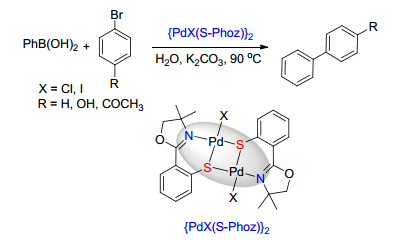
Guo, Y.; Li, J.; Shi, X.; Liu, Y.; Xie, K.; Liu, Y.; Jiang, Y.; Yang, B.; Yang, R. Appl. Organomet. Chem. 2017, 31, e3592. doi: 10.1002/aoc.3592
-
[32]
Khan, R. I.; Pitchumani, K. Green Chem. 2016, 18, 5518. doi: 10.1039/C6GC01326K
-
[33]
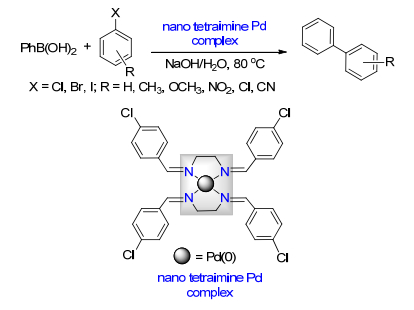
Ma, X.; Lv, G.; Cheng, X.; Li, W.; Sang, R.; Zhang, Y.; Wang, Q.; Hai, L.; Wu, Y. Appl. Organomet. Chem. 2017, 31, e3854. doi: 10.1002/aoc.3854
-
[34]
Shahnaz, N.; Puzari, A.; Paul, B.; Das, P. Catal. Commun. 2016, 86, 55. doi: 10.1016/j.catcom.2016.08.010
-
[35]
You, L.-X.; Liu, H.-J.; Cui, L.-X.; Ding, F.; Xiong, G.; Wang, S.-J.; Ren, B.-Y.; Dragutan, L.; Dragutan, V.; Sun, Y.-G. Dalton Trans. 2016, 45, 18455. doi: 10.1039/C6DT03628G
-
[36]
Fiebor, A.; Tia, R.; Makhubela, B. C. E.; Kinfe, H. H. Beilstein J. Org. Chem. 2018, 14, 1859. doi: 10.3762/bjoc.14.160
-
[37]
Ramakrishna, V.; Reddy N. D. Dalton Trans. 2017, 46, 8598. doi: 10.1039/C7DT01433C
-
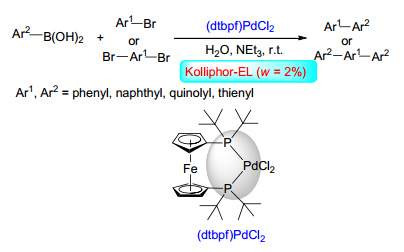
[38]
Scattolin, T.; Canovese, L.; Visentin, F.; Paganelli, S.; Canton, P.; Demitri, N. Appl. Organomet. Chem. 2017, e4034.
-
[39]
Lee, J.-Y.; Tzeng, R.-J.; Wang, M.-C.; Lee, H. M. Inorg. Chim. Acta 2017, 464, 74. doi: 10.1016/j.ica.2017.04.046
-
[40]
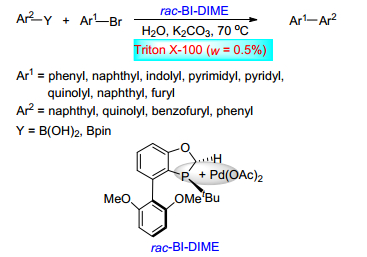
Qiu, P.; Zhao, J. Y.; Shi, X.; Duan, X. H. New J. Chem. 2016, 40, 6568. doi: 10.1039/C6NJ00377J
-
[41]
Xu, S. D.; Sun, F. Z.; Deng, W. H.; Hao, H.; Duan, X. H. New J. Chem. 2018, 42, 16464. doi: 10.1039/C8NJ02184H
-
[42]
Saito, S.; Sakai, M.; Miyaura, N. Tetrahedron Lett. 1996, 37, 2993. doi: 10.1016/0040-4039(96)00482-0
-
[43]
Handa, S.; Slack, E. D.; Lipshutz, B. H. Angew. Chem., Int. Ed. 2015, 54, 11994. doi: 10.1002/anie.201505136
-
[44]
Gurung, S. K.; Thapa, S.; Kafle, A.; Dickie, D. A.; Giri, R. Org. Lett. 2014, 16, 1264. doi: 10.1021/ol500310u
-
[45]
(a) Wang, L.; Xie, Y.-B.; Huang, N.-Y.; Yan, J.-Y.; Hu, W.-M.; Liu, M.-G.; Ding, M.-W. ACS Catal. 2016, 6, 4010.
(b) Wang, L.; Xie, Y.-B.; Huang, N.-Y.; Zhang, N.-N.; Li, D.-J.; Hu, Y.-L.; Liu, M.-G.; Lia, D.-S. Adv. Synth. Catal. 2017, 359, 779. -
[46]
Handa, S.; Smith, J. D.; Hageman, M. S.; Gonzalez, M.; Lipshutz, B. H. ACS Catal. 2016, 6, 8179. doi: 10.1021/acscatal.6b02809
-
[47]
Landstrom, E. B.; Handa, S.; Aue, D. H.; Gallou, F.; Lipshutz, B. H. Green Chem. 2018, 20, 3436. doi: 10.1039/C8GC01356J
-
[48]
Patel, N. D.; Rivalti, D.; Buono, F. G.; Chatterjee, A.; Qu, B.; Braith, S.; Desrosiers, J.-N.; Rodriguez, S.; Sieber, J. D.; Haddad, N.; Fandrick, K. R.; Lee, H.; Yee, N. K.; Busacca, C. A.; Senanayake, C. H. Asian J. Org. Chem. 2017, 6, 1285. doi: 10.1002/ajoc.201700137
-
[49]
Mattiello, S.; Rooney, M.; Sanzone, A.; Brazzo, P.; Sassi, M.; Beverina, L. Org. Lett. 2017, 19, 654. doi: 10.1021/acs.orglett.6b03817
-
[50]
Sajith, A. M.; Abdul Khader, K. K.; Muralidharan, A.; Ali Padusha, M. S.; Nagaswarupa, H. P. J. Heterocycl. Chem. 2015, 52, 1748. doi: 10.1002/jhet.2277
-
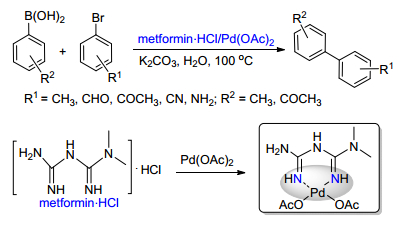
[51]
Fortun, S.; Beauclair P.; Schmitzer, A. R. RSC Adv. 2017, 7, 21036. doi: 10.1039/C7RA01197K
-
[52]
Kim, S.; Cho, H.-J.; Lee, N.; Lee, Y.-S.; Shin, D.-S.; Lee, S.-M. RSC Adv. 2017, 7, 33162. doi: 10.1039/C7RA04793B
-
[1]
-

-
-
[1]
Honghong Zhang , Zhen Wei , Derek Hao , Lin Jing , Yuxi Liu , Hongxing Dai , Weiqin Wei , Jiguang Deng . 非均相催化CO2与烃类协同催化转化的最新进展. Acta Physico-Chimica Sinica, 2025, 41(7): 100073-0. doi: 10.1016/j.actphy.2025.100073
-
[2]
Shiyi WANG , Chaolong CHEN , Xiangjian KONG , Lansun ZHENG , Lasheng LONG . Polynuclear lanthanide compound [Ce4ⅢCe6Ⅳ(μ3-O)4(μ4-O)4(acac)14(CH3O)6]·2CH3OH for the hydroboration of amides to amine. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 88-96. doi: 10.11862/CJIC.20240342
-
[3]
Xin Han , Zhihao Cheng , Jinfeng Zhang , Jie Liu , Cheng Zhong , Wenbin Hu . Design of Amorphous High-Entropy FeCoCrMnBS (Oxy) Hydroxides for Boosting Oxygen Evolution Reaction. Acta Physico-Chimica Sinica, 2025, 41(4): 2404023-0. doi: 10.3866/PKU.WHXB202404023
-
[4]
Kai CHEN , Fengshun WU , Shun XIAO , Jinbao ZHANG , Lihua ZHU . PtRu/nitrogen-doped carbon for electrocatalytic methanol oxidation and hydrogen evolution by water electrolysis. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1357-1367. doi: 10.11862/CJIC.20230350
-
[5]
Lili Jiang , Shaoyu Zheng , Xuejiao Liu , Xiaomin Xie . Copper-Catalyzed Oxidative Coupling Reactions for the Synthesis of Aryl Sulfones: A Fundamental and Exploratory Experiment for Undergraduate Teaching. University Chemistry, 2025, 40(7): 267-276. doi: 10.12461/PKU.DXHX202408004
-
[6]
Qingqing SHEN , Xiangbowen DU , Kaicheng QIAN , Zhikang JIN , Zheng FANG , Tong WEI , Renhong LI . Self-supporting Cu/α-FeOOH/foam nickel composite catalyst for efficient hydrogen production by coupling methanol oxidation and water electrolysis. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1953-1964. doi: 10.11862/CJIC.20240028
-
[7]
Sumiya Akter Dristy , Md Ahasan Habib , Shusen Lin , Mehedi Hasan Joni , Rutuja Mandavkar , Young-Uk Chung , Md Najibullah , Jihoon Lee . Exploring Zn doped NiBP microspheres as efficient and stable electrocatalyst for industrial-scale water splitting. Acta Physico-Chimica Sinica, 2025, 41(7): 100079-0. doi: 10.1016/j.actphy.2025.100079
-
[8]
Liu Lin , Zemin Sun , Huatian Chen , Lian Zhao , Mingyue Sun , Yitao Yang , Zhensheng Liao , Xinyu Wu , Xinxin Li , Cheng Tang . Recent Advances in Electrocatalytic Two-Electron Water Oxidation for Green H2O2 Production. Acta Physico-Chimica Sinica, 2024, 40(4): 2305019-0. doi: 10.3866/PKU.WHXB202305019
-
[9]
Meiran Li , Yingjie Song , Xin Wan , Yang Li , Yiqi Luo , Yeheng He , Bowen Xia , Hua Zhou , Mingfei Shao . Nickel-Vanadium Layered Double Hydroxides for Efficient and Scalable Electrooxidation of 5-Hydroxymethylfurfural Coupled with Hydrogen Generation. Acta Physico-Chimica Sinica, 2024, 40(9): 2306007-0. doi: 10.3866/PKU.WHXB202306007
-
[10]
Yuchen Zhou , Huanmin Liu , Hongxing Li , Xinyu Song , Yonghua Tang , Peng Zhou . Designing thermodynamically stable noble metal single-atom photocatalysts for highly efficient non-oxidative conversion of ethanol into high-purity hydrogen and value-added acetaldehyde. Acta Physico-Chimica Sinica, 2025, 41(6): 100067-0. doi: 10.1016/j.actphy.2025.100067
-
[11]
Huafeng SHI . Construction of MnCoNi layered double hydroxide@Co-Ni-S amorphous hollow polyhedron composite with excellent electrocatalytic oxygen evolution performance. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1380-1386. doi: 10.11862/CJIC.20240378
-
[12]
Nengmin ZHU , Wenhao ZHU , Xiaoyao YIN , Songzhi ZHENG , Hao LI , Zeyuan WANG , Wenhao WEI , Xuanheng CHEN , Weihai SUN . Preparation of high-performance CsPbBr3 perovskite solar cells by the aqueous solution solvent method. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1131-1140. doi: 10.11862/CJIC.20240419
-
[13]
Lijuan Liu , Xionglei Wang . Preparation of Hydrogels from Waste Thermosetting Unsaturated Polyester Resin by Controllable Catalytic Degradation: A Comprehensive Chemical Experiment. University Chemistry, 2024, 39(11): 313-318. doi: 10.12461/PKU.DXHX202403060
-
[14]
Xi YANG , Chunxiang CHANG , Yingpeng XIE , Yang LI , Yuhui CHEN , Borao WANG , Ludong YI , Zhonghao HAN . Co-catalyst Ni3N supported Al-doped SrTiO3: Synthesis and application to hydrogen evolution from photocatalytic water splitting. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 440-452. doi: 10.11862/CJIC.20240371
-
[15]
Yaping Li , Sai An , Aiqing Cao , Shilong Li , Ming Lei . The Application of Molecular Simulation Software in Structural Chemistry Education: First-Principles Calculation of NiFe Layered Double Hydroxide. University Chemistry, 2025, 40(3): 160-170. doi: 10.12461/PKU.DXHX202405185
-
[16]
Wentao Xu , Xuyan Mo , Yang Zhou , Zuxian Weng , Kunling Mo , Yanhua Wu , Xinlin Jiang , Dan Li , Tangqi Lan , Huan Wen , Fuqin Zheng , Youjun Fan , Wei Chen . Bimetal Leaching Induced Reconstruction of Water Oxidation Electrocatalyst for Enhanced Activity and Stability. Acta Physico-Chimica Sinica, 2024, 40(8): 2308003-0. doi: 10.3866/PKU.WHXB202308003
-
[17]
Yi Yang , Xin Zhou , Miaoli Gu , Bei Cheng , Zhen Wu , Jianjun Zhang . Femtosecond transient absorption spectroscopy investigation on ultrafast electron transfer in S-scheme ZnO/CdIn2S4 photocatalyst for H2O2 production and benzylamine oxidation. Acta Physico-Chimica Sinica, 2025, 41(6): 100064-0. doi: 10.1016/j.actphy.2025.100064
-
[18]
.
CCS Chemistry | 超分子活化底物为自由基促进高效选择性光催化氧化
. CCS Chemistry, 2025, 7(10.31635/ccschem.025.202405229): -. -
[19]
Yongmei Liu , Lisen Sun , Yongmei Hao , Zhanxiang Liu , Shuyong Zhang . Innovative Design of Chemistry Experiment Courses with Ideological and Political Education: A Case Study of Catalytic Hydrogen Production Experiments. University Chemistry, 2025, 40(5): 224-229. doi: 10.12461/PKU.DXHX202412144
-
[20]
Xiaogang Liu , Mengyu Chen , Yanyan Li , Xiantao Ma . Experimental Reform in Applied Chemistry for Cultivating Innovative Competence: A Case Study of Catalytic Hydrogen Production from Liquid Formaldehyde Reforming at Room Temperature. University Chemistry, 2025, 40(7): 300-307. doi: 10.12461/PKU.DXHX202408007
-
[1]
Metrics
- PDF Downloads(52)
- Abstract views(3062)
- HTML views(785)

 Login In
Login In




 DownLoad:
DownLoad: