Citation:
Liu Xueying, Liu Zhenwei, Guo Yuanyuan, Li Jingya, Zou Dapeng, Wu Yusheng, Wu Yangjie. One-Pot, Two-Step Reductive Amination of Boronate Ester Containing Aromatic Amines and Aldehydes Using B2pin2 as Reductant[J]. Chinese Journal of Organic Chemistry,
;2019, 39(7): 2001-2008.
doi:
10.6023/cjoc201902011

-
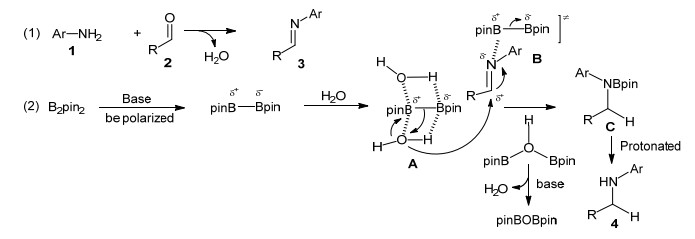
The aromatic amine functionality occupies a very important role in organic chemistry due to its prominence in biological and naturally occurring molecules. In addition, the synthesized secondary aromatic amines with pendant boronate ester are versatile intermediates in several organic transformations. The one-pot, two-step reductive amination of boron-containing primary aromatic amines and aldehydes has been achieved in the presence of NaOH in ethanol using B2pin2 as reductant. After extensive screening of various reaction parameters, such as base, reaction temperature, solvent, reaction time and protective gas, a series of secondary aromatic amines with pendant boronate ester and various functional groups were obtained in moderate to good yields under the optimal reaction conditions. This system features generally high yields and broad functional group tolerance. The boronate ester substituent is a very good handle to be further functionalized.
-

-
-
[1]
Pham, P. D.; Bertus, P.; Legoupy, S. Chem. Commun. 2009, 6207.
-
[2]
For literature pertaining to the origins and definition of reductive amination, see:(a) Emerson, W. S. Org. React. 1948, 4, 174.
(b) Moore, M. L. Org. React. 1949, 5, 301.
(c) Nugenta, T. C.; El-Shazly, M. Adv. Synth. Catal. 2010, 352, 753. -
[3]
Chi, Y.; Zhou, Y.; Zhang, X. J. Org. Chem. 2003, 68, 4120. doi: 10.1021/jo026856z
-
[4]
(a) Skucas, E.; Kong, J.; Krische, M. J. J. Am. Chem. Soc. 2007, 129, 7242.
(b) Park, J. W.; Chung, Y. K. ACS Catal. 2015, 5, 4846. -
[5]
Pagnoux-Ozherelyeva, A.; Pannetier, N.; Mbaye, M. D.; Gaillard, S.; Renaud, J.-L. Angew. Chem., Int. Ed. 2012, 51, 4976. doi: 10.1002/anie.201201360
-
[6]
Nasrollahzadeh, M. New J. Chem. 2014, 38, 5544. doi: 10.1039/C4NJ01440E
-
[7]
Huang, L.; Wang, Z.; Geng, L.; Chen, R.; Xing, W.; Wang, Y.; Huang, J. RSC Adv. 2015, 5, 56936. doi: 10.1039/C5RA05243B
-
[8]
Gao, G.; Sun, P.; Li, Y.; Wang, F.; Zhao, Z.; Qin, Y.; Li, F. ACS Catal. 2017, 7, 4927. doi: 10.1021/acscatal.7b01786
-
[9]
Abdel-Magid, A. F.; Carson, K. G.; Harris, B. D.; Maryanoff, C. A.; Shah, R. D. J. Org. Chem. 1996, 61, 3849. doi: 10.1021/jo960057x
-
[10]
Roe, A.; Montgomery, J. A. J. Am. Chem. Soc. 1953, 75, 910. doi: 10.1021/ja01100a040
-
[11]
Tripathi, R. P.; Verma, S. S.; Pandey, J.; Tiwari, V. K. Curr. Org. Chem. 2008, 12, 1093. doi: 10.2174/138527208785740283
-
[12]
(a) Borch, R. F.; Bernstein, M. D.; Durst, H. D. J. Am. Chem. Soc. 1971, 93, 2897.
(b) Borch, R. F.; Hassid, A. I. J. Org. Chem. 1972, 37, 1673.
(c) Marchini, P.; Liso, G.; Reho, A.; Liberatone, F.; Moracci, F. M. J. Org. Chem. 1975, 40, 3453.
(d) Lane, C. F. Synthesis 1975, 135. -
[13]
(a) Abdel-Magid, A. F.; Maryanoff, C. A.; Carson, K. G. Tetrahedron Lett. 1990, 31, 5595.
(b) Kim, H. O.; Carrol, B.; Lee, M. S. Synth. Commun. 1997, 27, 2505.
(c) Tarasevich, V. A.; Kozlov, N. G. Russ. Chem. Rev. 1999, 68, 55. -
[14]
Borch, R. F.; Durst, H. D. J. Am. Chem. Soc. 1969, 91, 3996. doi: 10.1021/ja01042a078
-
[15]
(a) Ros, A.; Fernandez, R.; Lassaletta, J. M. Chem. Soc. Rev. 2014, 43, 3229.
(b) Hartwig, J. F. Acc. Chem. Res. 2012, 45, 864.
(c) Hartwig, J. F. Chem. Soc. Rev. 2011, 40, 1992.
(d) Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110, 890. -
[16]
(a) Xu, H.; Zhao, C.; Qian, Q.; Deng, W.; Gong, H. Chem. Sci. 2013, 4, 4022.
(b) Yu, X.; Wang, S.; Xu, H.; Gong, H. Org. Lett. 2011, 13, 2138.
(c) Liang, Z.; Xue, W.; Lin, K.; Gong, H. Org. Lett. 2014, 16, 5620.
(d) Zhang, G.; Xie, Y.; Wang, Z.; Liu, Y.; Huang, H. Chem. Commun. 2015, 51, 1850.
(e) Ke, M.; Song, Q. J. Org. Chem. 2016, 81, 3654.
(f) Doi, R.; Ohashi, M.; Ogoshi, S. Angew. Chem., Int. Ed. 2016, 55, 341.
(g) Ke, M.; Song, Q. Chem. Commun. 2017, 53, 2222.
(h) Chen, Z.; Wang, X. Org. Biomol. Chem. 2017, 15, 5790.
(i) Ke, M.; Song, Q. Adv. Synth. Catal. 2017, 359, 384.
(j) Lu, X.; Wang, Y.; Zhang, B.; Pi, J.; Wang, X.; Gong, T.; Xiao, B.; Fu, Y. J. Am. Chem. Soc. 2017, 139, 12632.
(k) Kuang, Z.; Li, B.; Song, Q. Chem. Commun. 2018, 54, 34. -
[17]
(a) Ojha, D. P.; Gadde, K.; Prabhu, K. R. Org. Lett. 2016, 18, 5062.
(b) Ding, W.; Song, Q. Org. Chem. Front. 2016, 3, 14.
(c) Wang, Q.; Yang, J.; Fang, D.; Ren, J.; Dong, B.; Zhou, B.; Zeng, B. Tetrahedron Lett. 2016, 57, 2587. -
[18]
(a) Laitar, D. S.; Müller, P.; Sadighi, J. P. J. Am. Chem. Soc. 2005, 127, 17196.
(b) Bae, S.; Lakshman, M. K. J. Org. Chem. 2008, 73, 1311.
(c) Kokatla, H. P.; Thomson, P. F.; Bae, S.; Doddi, V. R.; Lakshman, M. K. J. Org. Chem. 2011, 76, 7842.
(d) Xuan, Q.; Zhao, C.; Song, Q. Org. Biomol. Chem. 2017, 15, 5140. -
[19]
(a) Lu, H.; Geng, Z.; Li, J.; Zou, D.; Wu, Y. S.; W, Y. J. Org. Lett. 2016, 18, 2774.
(b) Yang, K.; Zhou, F.; Kuang, Z.; Gao, G.; Driver, T. Org. Lett. 2016, 18, 4088. -
[20]
Enthaler, S. Catal. Lett. 2012, 142, 1306. doi: 10.1007/s10562-012-0897-y
-
[21]
Xuan, Q.; Song Q. Org. Lett. 2016, 18, 4250. doi: 10.1021/acs.orglett.6b01999
-
[22]
(a) Lu, H.; Wang, S.; Li, J.; Zou, D.; Wu, Y. S.; Wu, Y. J. Tetrahedron Lett. 2017, 58, 839.
(b) Zhi, W.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. J. Org. Chem. 2017, 82, 12286.
(c) Ren, X.; Han, S.; Gao, X.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 1065.
(d) Zhi, W.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 537.
(e) Zhi, W.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 2736. -
[23]
(a) Geng, Z.; Zhang, Y.; Zheng, L.; Li, J.; Zou, D.; Wu, Y. J.; Wu, Y. S. Tetrahedron Lett. 2016, 57, 3063.
(b) Zhang, Y.; Geng, Z.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Adv. Synth. Catal. 2017, 359, 390.
(c) Zhu, M.; Qiu, Z.; Zhang, Y.; Du, H.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2017, 58, 2255.
(d) Zhu, M.; Du, H.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 1352. -
[24]
(a) Menet, C. J. M.; Blanc, J.; Hodges, A. J.; Burli, R. W.; Breccia, P.; Blackaby, W. P.; Van Rompaey, L. J. C.; Fletcher, S. R. WO 2010/010184, 2010.
(b) Liu, B.; Huang, J.; Zheng, C.; Zhang, Y.; Ouyang, L.; Mao, H.; Nie, B.; Xu, J.; Chen, H. CN 2015/10402418, 2015. -
[25]
Chen, Y.; Liu, S.; Cui, P.; Zhang, J.; Liu, Q.; Zhou, H. Tetrahedron Lett. 2019, 60, 327. doi: 10.1016/j.tetlet.2018.12.041
-
[26]
Christopher, M. V.; Liliya, G. N.; David, W. N.; Heather, A. S.; Andreas, D.; Mark, O. B.; Felix, J. B.; Stephen, A. W. Can. J. Chem. 2001, 79, 1115. doi: 10.1139/v01-090
-
[27]
Wheaton, S. L.; Humanayun Kabir, S. M.; Zhang, H.; Vogels1, C. M.; Decken, A.; Westcott, S. Cent. Eur. J. Chem. 2010, 8, 725.
-
[1]
-

-
-
[1]
Xiuhua Wang , Jianrong Steve Zhou . A dicationic nickel complex-catalyzed asymmetric synthesis of chiral benzylic amines: Evolution from reductive amination to borrowing hydrogen reaction. Chinese Chemical Letters, 2026, 37(2): 111148-. doi: 10.1016/j.cclet.2025.111148
-
[2]
Wen-Tao Ouyang , Jun Jiang , Yan-Fang Jiang , Ting Li , Yuan-Yuan Liu , Hong-Tao Ji , Li-Juan Ou , Wei-Min He . Sono-photocatalytic amination of quinoxalin-2(1H)-ones with aliphatic amines. Chinese Chemical Letters, 2024, 35(10): 110038-. doi: 10.1016/j.cclet.2024.110038
-
[3]
Lei Shen , Yang Zhang , Linlin Zhang , Chuanwang Liu , Zhixian Ma , Kangjiang Liang , Chengfeng Xia . Phenylhydrazone anions excitation for the photochemical carbonylation of aryl iodides with aldehydes. Chinese Chemical Letters, 2024, 35(4): 108742-. doi: 10.1016/j.cclet.2023.108742
-
[4]
Kun Tang , Fen Su , Shijie Pan , Fengfei Lu , Zhongfu Luo , Fengrui Che , Xingxing Wu , Yonggui Robin Chi . Enones from aldehydes and alkenes by carbene-catalyzed dehydrogenative couplings. Chinese Chemical Letters, 2024, 35(9): 109495-. doi: 10.1016/j.cclet.2024.109495
-
[5]
Jun Zhang , Zhiyao Zheng , Can Zhu . Stereochemical editing: Catalytic racemization of secondary alcohols and amines. Chinese Chemical Letters, 2024, 35(5): 109160-. doi: 10.1016/j.cclet.2023.109160
-
[6]
Zhikang Wu , Guoyong Dai , Qi Li , Zheyu Wei , Shi Ru , Jianda Li , Hongli Jia , Dejin Zang , Mirjana Čolović , Yongge Wei . POV-based molecular catalysts for highly efficient esterification of alcohols with aldehydes as acylating agents. Chinese Chemical Letters, 2024, 35(8): 109061-. doi: 10.1016/j.cclet.2023.109061
-
[7]
Yi-Fan Wang , Hao-Yun Yu , Hao Xu , Ya-Jie Wang , Xiaodi Yang , Yu-Hui Wang , Ping Tian , Guo-Qiang Lin . Rhodium(Ⅲ)-catalyzed diastereo- and enantioselective hydrosilylation/cyclization reaction of cyclohexadienone-tethered α, β-unsaturated aldehydes. Chinese Chemical Letters, 2024, 35(9): 109520-. doi: 10.1016/j.cclet.2024.109520
-
[8]
Jinyuan Cui , Tingting Yang , Teng Xu , Jin Lin , Kunlong Liu , Pengxin Liu . Hydrogen spillover enhances the selective hydrogenation of α,β-unsaturated aldehydes on the Cu-O-Ce interface. Chinese Journal of Structural Chemistry, 2025, 44(1): 100438-100438. doi: 10.1016/j.cjsc.2024.100438
-
[9]
Jiaxuan YANG , Chenfa DENG , Jingyang LIU , Chenzexi XU , Hongxin CHEN , Yahui ZHU , Ying LI , Shuhua WANG , Rongping ZHOU , Chao CHEN . Advances in selective hydrogenation of α, β-unsaturated aldehydes/ketones catalyzed by metal-organic frameworks and their derivatives: A review. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 1973-2010. doi: 10.11862/CJIC.20250175
-
[10]
Hua Liu , Jian Zhao , Qi Li , Xiang-Yu Zhang , Zhi-Wei Zheng , Kun Huang , Da-Bin Qin , Bin Zhao . Indium-captured zirconium-porphyrin frameworks displaying rare multi-selectivity for catalytic transfer hydrogenation of aldehydes and ketones. Chinese Chemical Letters, 2025, 36(6): 110593-. doi: 10.1016/j.cclet.2024.110593
-
[11]
Pengcheng Su , Shizheng Chen , Zhihong Yang , Ningning Zhong , Chenzi Jiang , Wanbin Li . Vapor-phase postsynthetic amination of hypercrosslinked polymers for efficient iodine capture. Chinese Chemical Letters, 2024, 35(9): 109357-. doi: 10.1016/j.cclet.2023.109357
-
[12]
Xiao-Bo Liu , Ren-Ming Liu , Xiao-Di Bao , Hua-Jian Xu , Qi Zhang , Yu-Feng Liang . Nickel-catalyzed reductive formylation of aryl halides via formyl radical. Chinese Chemical Letters, 2024, 35(12): 109783-. doi: 10.1016/j.cclet.2024.109783
-
[13]
Saima Perveen , Xicheng Wang , Tao Li , Linghua Wang , Shuai Zhang , Yizhao Ouyang , Xue Zhao , Liang Xu , Pengfei Li . Enantioconvergent reductive amidation of benzyl ammonium salts for synthesis of α-chiral amides. Chinese Chemical Letters, 2026, 37(1): 111779-. doi: 10.1016/j.cclet.2025.111779
-
[14]
Chong-Yang Shi , Jian-Xing Gong , Zhen Li , Chao Shu , Long-Wu Ye , Qing Sun , Bo Zhou , Xin-Qi Zhu . Gold-catalyzed intermolecular amination of allyl azides with ynamides: Efficient construction of 3-azabicyclo[3.1.0] scaffold. Chinese Chemical Letters, 2025, 36(2): 109895-. doi: 10.1016/j.cclet.2024.109895
-
[15]
Wen-Jing Li , Jun-Bo Wang , Yu-Heng Liu , Mo Zhang , Zhan-Hui Zhang . Molybdenum-doped carbon nitride as an efficient heterogeneous catalyst for direct amination of nitroarenes with arylboronic acids. Chinese Chemical Letters, 2025, 36(3): 110001-. doi: 10.1016/j.cclet.2024.110001
-
[16]
Haoquan Huang , Haiting Chen , Xinran Dong , Yanbin Xu , Anlian Huang , Qiaoyi Cen , Huairou Zhu , Guosheng Chen , Wei Yi , Siming Huang , Gangfeng Ouyang . Site-specific surface amination strategy facilitates biomimetic encapsulation of enzymes within hydrogen-bonded organic framework. Chinese Chemical Letters, 2025, 36(9): 111223-. doi: 10.1016/j.cclet.2025.111223
-
[17]
Tingting Huang , Zhuanlong Ding , Hao Liu , Ping-An Chen , Longfeng Zhao , Yuanyuan Hu , Yifan Yao , Kun Yang , Zebing Zeng . Electron-transporting boron-doped polycyclic aromatic hydrocarbons: Facile synthesis and heteroatom doping positions-modulated optoelectronic properties. Chinese Chemical Letters, 2024, 35(4): 109117-. doi: 10.1016/j.cclet.2023.109117
-
[18]
Junxin Li , Chao Chen , Yuzhen Dong , Jian Lv , Jun-Mei Peng , Yuan-Ye Jiang , Daoshan Yang . Ligand-promoted reductive coupling between aryl iodides and cyclic sulfonium salts by nickel catalysis. Chinese Chemical Letters, 2024, 35(11): 109732-. doi: 10.1016/j.cclet.2024.109732
-
[19]
Li Li , Zhi-Xin Yan , Chuan-Kun Ran , Yi Liu , Shuo Zhang , Tian-Yu Gao , Long-Fei Dai , Li-Li Liao , Jian-Heng Ye , Da-Gang Yu . Electro-reductive carboxylation of CCl bonds in unactivated alkyl chlorides and polyvinyl chloride with CO2. Chinese Chemical Letters, 2024, 35(12): 110104-. doi: 10.1016/j.cclet.2024.110104
-
[20]
Zhen-Zhen Dong , Jin-Hao Zhang , Lin Zhu , Xiao-Zhong Fan , Zhen-Guo Liu , Yi-Bo Yan , Long Kong . Attenuating reductive decomposition of fluorinated electrolytes for high-voltage lithium metal batteries. Chinese Chemical Letters, 2025, 36(4): 109773-. doi: 10.1016/j.cclet.2024.109773
-
[1]
Metrics
- PDF Downloads(9)
- Abstract views(1422)
- HTML views(116)

 Login In
Login In




 DownLoad:
DownLoad:
