 图1
Biologically active carbazole derivatives
Figure1.
Biologically active carbazole derivatives
图1
Biologically active carbazole derivatives
Figure1.
Biologically active carbazole derivatives

钯催化取代吲哚与苯丙酮类化合物的直接偶联构筑咔唑衍生物
English
Construction of Carbazoles by Palladium-Catalyzed Direct Cross-Coupling of Indoles with in situ Generated Aryl Vinyl Ketones
-
Key words:
- palladium-catalyzed
- / dehydrogenation
- / carbazoles
- / C—H functionalization
- / cross-coupling
-
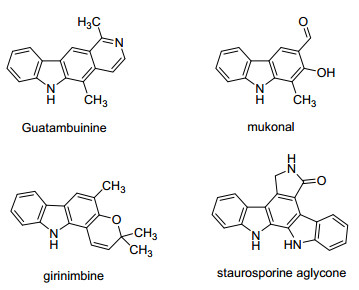
Carbazoles represent an important class of nitrogen-containing heterocyclic compounds, which are ubiquitous as structural motifs in various natural products, pharmaceuticals and biologically active compounds (Figure 1).[1] Traditionally, the most commonly used method for preparation of this kind of molecular framework mainly relies upon nitrene insertion, Fischer indolization, Pummerer cyclization, Dielse-Alder reaction, dehydrogenative cyclization of diarylamines, etc.[2] In view of their significant importance and wide application, synthetic organic chemists are devoted to seek efficient methods for the construction of carbazoles during the past several decades.
The past decades have witnessed the rapid development of transition metal-catalyzed C—H bond functionalization reactions, which have evolved into a powerful tool for the synthesis of numerous structurally complex molecules from simple starting materials.[3] Several methods involving C—H cleavage and C—C/C―N formation have been used for synthesis of carbazoles, such as oxidative cyclization of biarylamines[4] and intramolecular C—H amination of anilides.[5] Among the various methods developed in recent years, the indole-to-carbazole transformation, which relies upon the construction of a benzene ring into indoles, has attracted widespread attention since it offers a direct and convenient approach to carbazoles from readily available indoles. Substituted carbazoles were obtained via the [2+ 2+2] cycloaddition of indoles with alkyne.[6] Recently, the synthesis of carbazoles by direct cross-coupling of indoles with alkenes has appeared, [7] and those reactions were proposed to occur via an initially alkenylation of indoles, [8] followed by a Diels-Alder reaction of the 3-alkenyated indoles with the second alkenes.
Although there are reports on the direct C—H/C—H coupling of indoles with alkenes to provide carbazoles, examples using aryl vinyl ketones are much less common. In this context, Yu and coworkers[9] reported the synthesis of 1, 3-disubstituted carbazoles via the reactions of indoles with in-situ generated aryl vinyl ketones using saturated β-chloroalkyl aryl ketones as the olefins source. Aryl vinyl ketones are not commercially available, typical preparation of aryl vinyl ketones require multistep preparation routes[10] and/or use of stoichiometric reagents such as 2-iodoxy-benzoic acid (IBX)[11] and dichloro-5, 6-dicyano-1, 4-ben-zoquinone (DDQ).[12] The Pd-catalyzed oxidative dehydrogenation of ketones represents a promising method for the facile synthesis of α, β-unsaturated ketones, but is still limited to cyclic enones or β-arylated enones.[13] Consequently, the reactions that combine in situ dehydrogenation to form reactive aryl vinyl ketone intermediates with secondary coupling process are attracting attention from synthetic chemists. Herein, we report an efficient method for the facile syntheses of carbazoles via Pd-catalyzed dehydrogenative cross-coupling of indoles with in situ generated aryl vinyl ketones by using statured ketones as the olefins source.
As our ongoing interest in the development of C—H functionalization reactions, [14] we previously have reported several methods for palladium-catalyzed dehydrogenative olefination of (hetero)arenes via olefin intermediates generated in situ from saturated ketones and nitroalkanes.[15] Furthermore, we have achieved the β-functionalization of saturated ketones by palladium-catalyzed dehydorgenation of ketones in combination with subsequent conjugate addition.[16] These encouraging achievements led us to consider whether the dehydrogenative cross-coupling between indoles and saturated ketones could directly afford carbazoles. The expected transformation which just involves multiple successive C—H cleavages and C—C formation steps in one pot would provide a fundamentally new approach to carbazoles in an atom-and step-economical fashion.
1 Results and discussion
We initially screened a variety of reaction parameters using the reaction of N-methyl indole (1a) and propiophenone (2a) as a model system (Table 1). To our delight, the desired N-methyl-1, 3-dibenzoylcarbazole (3a) was obtained in 77% yield when the reaction mixture of 1a and 2a (10 equiv.) in 1, 2-dimethoxyethane (DME) was treated with Pd(OAc)2 (10 mol%), PCy3 (25 mol%), Ag2CO3 (3.0 equiv.), LiOAc (1.0 equiv.) and TEMPO (2, 2, 6, 6-tetra-methylpiperidine-N-oxyl) (0.4 equiv.) at 110 ℃ under 101 kPa of O2. Control experiments indicated that all the reaction parameters (palladium, copper, ligand, base and additive) were essential to furnish the product in high yield (Entries 2~6). The use of TEMPO (0.4 equiv.) as a co-oxidant led to an increase in yield presumably owing to its ability to accelerate reoxidation of Pd(0) to Pd(II) (Entry 4 vs 1). The yield were decreased if the amount of Ag2CO3 were reduced (Entries 7~8). Replacing PCy3 with other phosphine ligand such as PPh3, XPhos or Davephos significantly reduced the efficiency of reaction (Entries 9~11). LiOAc also played an irreplaceable role in enhancing the efficiency of the reaction (Entries 12~15). The yield was decreased significant when this reaction was carried out under air or N2 atmospher, indicating that O2 played a significant role as the oxidant in this reaction (Entries 16~17).

Entry Ag2CO3/ equiv. Ligand Base TEMPO/ equiv. Yieldb /% of3a 1 3.0 PCy3 LiOAc 0.4 77 (76) 2c 3.0 PCy3 LiOAc 0.4 0 3 0 PCy3 LiOAc 0.4 34 4 3.0 PCy3 LiOAc 0.0 26 5 3.0 LiOAc 0.4 Trace 6 3.0 PCy3 0.4 28 7 1.0 PCy3 LiOAc 0.4 45 8 2.0 PCy3 LiOAc 0.4 58 9 3.0 PPh3 LiOAc 0.4 Trace 10 3.0 Xphos LiOAc 0.4 Trace 11 3.0 Davephos LiOAc 0.4 Trace 12 3.0 PCy3 NaOAc 0.4 15 13 3.0 PCy3 KOAc 0.4 16 14 3.0 PCy3 Na2CO3 0.4 10 15 3.0 PCy3 K2CO3 0.4 Trace 16d 3.0 PCy3 LiOAc 0.4 50 17e 3.0 PCy3 LiOAc 0.4 25 aReaction conditons: 1a (0.2 mmol), 2a (10 equiv.), Pd(OAc)2 (10 mol%), ligand(25 mol%), Ag2CO3 (3.0 equiv.), base (1.0 equiv.), TEMPO (0.4 equiv.), 101 kPa O2, DME, 110 ℃, 24 h.; bNMR yield using CH2Br2 as internal standard (values in parentheses refers to the isolated yield); cin absence of Pd(TFA)2.; d the reaction was conducted under air.; ethe reaction was conducted under N2 atmosphere. With the optimized conditions in hand, we examined the substrate scope of this reaction with respect to propiophenones. As shown in Table 2, an array of substituted propiophenones underwent smoothly the reaction with N-methylindole (1a) to produce the desired 1, 3-disubstituted carbazoles in moderate yields. As a ligand, small adjustments about the amount of PCy3 were required to the reaction conditions in some cases to get better yields (3f, 3k). The variety of substituents tolerated in these cross-cou-plings, such as alkyl (3a~3e, 3g), alkoxy (3f, 3h), fluoro (3j), chloro (3k) and trifluoromethyl (3i) provide opportunities for further synthetic elaboration. Unfortunately, other sensitive substituents such as the ester, nitro, and cyano group were ineffective under the established conditions. Notably, heteroaromatic ketones such as 2-propionylthi-ophene were also appropriate reactants for this transformation (3l).
Next, we evaluated the scope of indoles by using propiophenone (2a) as a coupling partner. As shown in Table 3, the present protocol also proved to be applicable to N-Bn and N-n-Pr indoles (4a, 4b). However, when N-H indoles were induced to react with 2a under the established conditions, the desired products were not obtained. The reaction of indoles bearing electron-donating methyl and mothoxyl (4c, 4d), or electron-withdrawing fluoro and chloro (4e, 4f) substituents on benzene ring underwent the reaction smoothly to provide the desired products. Other electron-withdrawing substituents such as the ester, nitro, and cyano group were ineffective.
To probe the potential intermediates in this transformation, we examined the behavior of propiophenone (2a) in absence of indoles under standard conditions, which affored phenyl vinyl ketone (5) in 28% yield (Eq. 1). Moreover, the reaction of N-methyl indole (1a) with prepared phenyl vinyl ketone (5) provided desired product (3a) in 90% yield (Eq. 2). These observations provided evidence supporting that dehydrogenation of propiophenones is a key step lying in the catalytic cycle of this reaction.
Based on above results and our related work on dehydrogenation of ketones, a proposed mechanism for this reaction is shown in Scheme 1. This reaction may involve Pd-mediated C—H cleavage of indoles 1 to form a palladium intermediate A. Meanwhile, α, β-unsaturated ketones formed via dehydrogenation of propiophenones 2. The intermediate A undergoes a Heck-type reaction with α, β-unsaturated ketone to produce a C(3)-alkenylated indole intermediate C and a Pd(0) species. The Diels-Alder reaction of the 3-alkenylindole C with the second α, β-unsatu-rated ketone, and the subsequent dehydrogenative aromatization (oxidation) of the tetrahydrocarbazole intermedi-ates produce 1, 3-disubstituted carbazoles 3. The oxidant Ag2CO3/TEMPO/O2 facilitate the regeneration of Pd(II) catalyst.
2 Conclusions
In conclusion, a Pd-catalyzed direct cross-coupling reac-tion of indoles with ketones has been developed for the synthesis of carbazoles that are an important class of biologically active compounds. This protocol obviates the need for additional preparation steps of α, β-unsaturated ketones and therefore opens up a new door to construction of carbazoles. Further studies to expand the substrate scope of this transformation to other compound are ongoing in our laboratory.
3 Experimental section
3.1 Instruments and reagents
1H NMR, 13C NMR and 19F NMR spectra were recorded at 400, 100.6 and 376.5 MHz, respectively. High resolution mass spectra (HRMS) were determined on a Waters Micromass GCT Premier Mass Spectrometer at Shanghai institute of organic chemistry. All Reactions were performed under nitrogen N2with dry solvents under anhydrous conditions. DME was distilled from Na under a nitrogen atmosphere and stored as such. The Reagents and solvents used for experiments were purchased from Sigma-Aldrich, Acros Organics, TCI, and Alfa Aesar and used as received unless otherwise noted. 1-Benzyl-1H-indole, 1-propyl-1H-indole, 1, 5-dimethyl-1H-indole, 5-methoxy-1-methyl-1H-indole, 5-fluoro-1-methyl-1H-indole and 6-chloro-1-methyl-1H-indole were prepared according to procedures reported in the literatures.[17, 18, 19, 20]
3.2 Experimental method
In a glove box, a 25 mL of Schlenk tube equipped with a stir bar was charged with N-methylindoles (0.2 mmol), Pd(OAc)2 (0.002 mmol, 0.1 equiv.), PCy3 (0.005, 0.25 equiv.), LiOAc (0.2 mmol, 1.0 equiv.), TEMPO (0.08 mmol, 0.4 equiv.), Ag2CO3 (0.6 mmol, 3 equiv.). The tube was fitted with a rubber septum and removed out of the glove box. Propiophenone (2.0 mmol, 10.0 equiv.) and DME (1 mL) were added in turn to the Schlenk tube through the rubber septum using syringes. The tube was subjected to a vacuum and refilled with an O2 flow for three times, and then the septum was replaced with a Teflon screwcap under O2 flow (if the thiophene or the substituted propiophenone was solid, it was added to the tube in the glove box). The reaction mixture was stirred at 110 ℃ for 24 h. After cooling down, the reaction mixture was diluted with 10 mL of ethyl ether, filtered through a pad of silica gel, followed by washing the pad of the silica gel with the same solvent (20 mL), concentrated under reduced pressure. The residue was then purified by flash chromatography on silica gel with 2%~15% ethyl ether in petroleum ether as eluent to provide the corresponding products.
(9-Methyl-9H-carbazole-1, 3-diyl)bis(phenylmethanone) (3a)[7a]: White solid, 59.8 mg, 76%. 1H NMR (400 MHz, CDCl3) δ: 8.77 (s, 1H), 8.15 (d, J=7.7 Hz, 1H), 8.06 (s, 1H), 7.99 (d, J=7.6 Hz, 2H), 7.85 (d, J=7.3 Hz, 2H), 7.67~7.43 (m, 8H), 7.34 (t, J=7.3 Hz, 1H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.68, 195.66, 142.50, 140.99, 138.25, 137.56, 133.70, 133.33, 131.97, 130.58, 129.83, 129.69, 128.67, 128.60, 128.23, 127.97, 127.31, 127.15, 125.49, 124.63, 122.59, 122.27, 120.70, 120.46, 109.49, 33.09.
(9-Methyl-9H-carbazole-1, 3-diyl)bis(p-tolylmethanone) (3b)[9]: White solid, 44.0 mg, 53%. 1H NMR (400 MHz, CDCl3) δ: 8.77 (s, 1H), 8.17 (d, J=7.5 Hz, 1H), 8.05 (s, 1H), 7.90 (d, J=7.5 Hz, 2H), 7.79 (d, J=7.5 Hz, 2H), 7.57 (t, J=7.2 Hz, 1H), 7.47 (d, J=7.8 Hz, 1H), 7.39~7.26 (m, 5H), 3.69 (s, 3H), 2.47 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 195.54, 144.78, 142.70, 142.47, 140.80, 135.54, 135.12, 130.76, 130.12, 129.40, 129.35, 128.94, 127.69, 127.05, 125.19, 124.50, 122.64, 122.47, 120.57, 120.45, 109.43, 32.96, 21.72, 21.58.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((4-ethylphenyl)-methanone) (3c): White solid, 40.1 mg, 45%. 1H NMR (400 MHz, CDCl3) δ: 8.74 (d, J=1.6 Hz, 1H), 8.16 (d, J=7.7 Hz, 1H), 8.02 (d, J=1.5 Hz, 1H), 7.90 (d, J=8.2 Hz, 2H), 7.79 (d, J=8.1 Hz, 2H), 7.58~7.54 (m, 1H), 7.46 (d, J=8.2 Hz, 1H), 7.34~7.31 (m, 5H), 3.68 (s, 3H), 2.75 (q, J=7.6 Hz, 4H), 1.32~1.27 (m, 6H); 13C NMR (100 MHz, CDCl3) δ: 195.65, 150.95, 148.93, 142.52, 140.86, 135.80, 135.37, 130.94, 130.27, 129.50, 128.26, 127.80, 127.72, 127.08, 125.27, 124.54, 122.70, 122.53, 120.61, 120.52, 109.47, 33.03, 29.03, 28.93, 15.25, 15.15. HRMS (ESI) calcd for C31H28NO2 [M+H]+: 446.2115, found 446.2109.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((4-iso-propylphen-yl)methanone) (3d): White solid, 50.1 mg, 53%.1H NMR (400 MHz, CDCl3) δ: 8.79 (s, 1H), 8.19 (d, J=7.6 Hz, 1H), 8.07 (s, 1H), 7.95 (d, J=7.9 Hz, 2H), 7.84 (d, J=7.8 Hz, 2H), 7.58 (t, J=7.5 Hz, 1H), 7.49 (d, J=8.1 Hz, 1H), 7.39~3.76 (m, 5H), 3.72 (s, 3H), 3.03 (m, 2H), 1.33 (m, 12H); 13C NMR (100 MHz, CDCl3) δ: 195.54, 195.51, 155.40, 153.43, 142.49, 140.86, 135.93, 135.49, 130.96, 130.26, 129.58, 127.65, 127.04, 126.80, 126.36, 125.25, 124.53, 122.67, 122.51, 120.57, 120.48, 109.44, 34.32, 34.20, 33.01, 23.70, 23.60. HRMS (ESI) calcd for C33H32NO2 [M+H]+: 474.2428, found 474.2421.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((4-tert-butylphen-yl)methanone) (3e)[9]: White solid, 52.2 mg, 52%. 1H NMR (400 MHz, CDCl3) δ: 8.77 (d, J=1.4 Hz, 1H), 8.17 (d, J=7.7 Hz, 1H), 8.05 (d, J=1.3 Hz, 1H), 7.93 (d, J=8.4 Hz, 2H), 7.82 (d, J=8.3 Hz, 3H), 7.60~7.45 (m, 6H), 7.34 (t, J=7.4 Hz, 1H), 3.70 (s, 3H), 1.38 (d, J=3.7 Hz, 18H); 13C NMR (100 MHz, CDCl3) δ: 195.48, 157.60, 155.65, 142.49, 140.89, 135.49, 135.02, 130.67, 129.98, 129.66, 127.58, 127.04, 125.64, 125.28, 125.21, 124.55, 122.67, 122.46, 120.57, 120.48, 109.44, 35.19, 35.02, 33.02, 31.11, 31.02.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((4-methoxyphen-yl)methanone) (3f)[7a]: PCy3 (20 mol%). White solid, 51.5 mg, 57%. 1H NMR (400 MHz, CDCl3) δ: 8.71 (d, J=1.5 Hz, 1H), 8.16 (d, J=7.7 Hz, 1H), 7.97~7.95 (m, 2H), 7.93 (s, 1H), 7.86 (d, J=8.8 Hz, 2H), 7.56 (t, J=7.7, Hz 1H), 7.45 (d, J=8.2 Hz, 1H), 7.34 (t, J=7.4, 1H), 6.97 (dd, J=8.7 Hz, 6.5 Hz, 4H), 3.89 (s, 3H), 3.89 (s, 3H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 194.81, 194.68, 164.10, 162.85, 142.45, 140.60, 133.03, 132.38, 130.82, 130.59, 129.06, 128.00, 127.04, 124.85, 124.44, 122.65, 122.52, 120.52, 120.49, 113.96, 113.54, 109.42, 55.58, 55.47, 32.86.
(9-Methyl-9H-carbazole-1, 3-diyl)bis(m-tolylmethanone) (3g): White solid, 51.3 mg, 61%. 1H NMR (400 MHz, CDCl3) δ: 8.78 (d, J=1.4 Hz, 1H), 8.16 (d, J=7.7 Hz, 1H), 8.02 (d, J=1.0 Hz, 1H), 7.83 (s, 1H), 7.75 (d, J=7.6 Hz, 1H), 7.67 (s, 1H), 7.62 (d, J=6.7 Hz, 1H), 7.57 (t, J=7.7 Hz, 1H), 7.46 (dd, J=7.7 Hz, 4.8 Hz, 2H), 7.41~7.35 (m, 4H), 3.69 (s, 3H), 2.43 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 195.96, 142.50, 140.99, 138.61, 138.33, 138.14, 137.65, 134.54, 132.75, 130.84, 130.27, 129.66, 128.50, 128.14, 128.00, 127.44, 127.14, 127.12, 125.40, 124.66, 122.64, 122.33, 120.66, 120.49, 109.47, 33.08, 21.36, 21.31. HRMS (ESI) calcd for C29H24NO2 [M+H]+: 418.1802, found 418.1796.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((3-methoxyphen-yl)methanone) (3h): White solid, 89.8 mg, 58%. 1H NMR (400 MHz, CDCl3) δ: 8.77 (d, J=1.4 Hz, 1H), 8.16 (d, J=7.7 Hz, 1H), 8.05 (d, J=1.4 Hz, 1H), 7.58 (s, 1H), 7.55 (d, J=7.5 Hz, 1H), 7.46 (d, J=8.0 Hz, 2H), 7.41~7.34 (m, 5H), 7.19 (dd, J=8.1 Hz, 2.0 Hz, 1H), 7.15~7.10 (m, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 3.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.52, 195.47, 159.88, 159.53, 142.50, 141.02, 139.60, 138.94, 129.73, 129.63, 129.16, 127.30, 127.17, 125.50, 124.64, 123.92, 122.63, 122.53, 122.24, 120.73, 120.51, 120.39, 118.42, 114.20, 114.03, 109.50, 55.47, 55.40, 33.12. HRMS (ESI) calcd for C29H24NO4 [M+H]+: 450.1700, found 450.1695.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((3-(trifluorometh-yl)phenyl)methanone) (3i): White solid, 67.5 mg, 63%. 1H NMR (400 MHz, CDCl3) δ: 8.78 (d, J=1.4 Hz, 1H), 8.34 (s, 1H), 8.15 (t, J=8.1 Hz, 2H), 8.11 (s, 1H), 7.99 (d, J=8.6 Hz, 2H), 7.92 (d, J=7.8 Hz, 1H), 7.84 (d, J=7.8 Hz, 1H), 7.70~7.57 (m, 3H), 7.50 (d, J=8.2 Hz, 1H), 7.38 (t, J=7.4 Hz, 1H), 3.72 (s, 3); 13C NMR (100 MHz, CDCl3) δ: 193.89, 193.71, 142.67, 141.41, 138.87, 138.10, 133.90, 132.81, 130.08 (q, J=3.5 Hz), 129.75, 129.44, 128.90, 128.50 (q, J=3.5 Hz), 127.60, 126.94 (q, J=3.8 Hz), 126.44 (q, J=4.1 Hz), 125.93, 125.36, 122.46, 121.37, 121.16, 120.57, 109.71, 33.39; 19F NMR (376 MHz, CDCl3) δ: -62.66 (s, 3F), -62.70 (s, 3F). HRMS (ESI) calcd for C29H18F6NO2 [M+H]+: 526.1234, found 526.1230.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((2-fluorophenyl)-methanone) (3j): White solid, 67.5 mg, 63%. 1H NMR (400 MHz, CDCl3) δ: 8.72 (d, J=1.3 Hz, 1H), 8.17~8.10 (m, 2H), 7.84 (td, J=7.5 Hz, 1.7 Hz, 1H), 7.67~7.50 (m, 5H), 7.38~7.30 (m, 3H), 7.24~7.14 (m, 2H), 3.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 192.23, 191.94, 161.84 (d, J=255.3 Hz), 159.31 (d, J=255.2 Hz), 134.83 (d, J=8.8 Hz), 132.73 (d, J=8.2 Hz), 131.98, 130.63 (d, J=2.9 Hz), 129.98, 127.38, 127.33, 127.27, 127.17, 126.80 (d, J=11.0 Hz), 126.16, 125.21, 124.44 (d, J=3.8 Hz), 124.23 (d, J=3.6 Hz), 123.83, 122.64, 120.99, 120.43, 116.80 (d, J=21.9 Hz), 116.17 (d, J=21.8 Hz), 109.76, 33.83. 19F NMR (376 MHz, CDCl3) δ: -109.44 (s, 1F), -111.34 (s, 1F). HRMS (ESI) calcd for C27H18F2NO2 [M+H]+: 426.1300, found 426.1296.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((3-chlorophenyl)-methanone) (3k): PCy3 (20 mol%). White solid, 45.7 mg, 50%. 1H NMR (400 MHz, CDCl3) δ: 8.76 (s, 1H), 8.17 (d, J=7.7 Hz, 1H), 7.99 (d, J=11.7 Hz, 2H), 7.82 (d, J=8.9 Hz, 2H), 7.68 (d, J=7.5 Hz, 1H), 7.64~7.54 (m, 3H), 7.51~7.42 (m, 3H), 7.38 (t, J=7.4 Hz, 3H), 3.71 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 194.08, 193.99, 142.63, 141.26, 139.89, 139.14, 135.16, 134.62, 133.69, 132.04, 130.28, 130.07, 129.84, 129.70, 129.61, 128.91, 127.89, 127.50, 126.68, 125.81, 125.13, 122.52, 121.55, 121.05, 120.61, 109.67, 33.36. HRMS (ESI) calcd for C27H18-Cl2NO2 [M+H]+: 458.0704, found 458.0704.
(9-Methyl-9H-carbazole-1, 3-diyl)bis((5-methylthioph-en-2-yl)methanone) (3l): White solid, 47.2 mg, 55%. 1H NMR (400 MHz, CDCl3) δ: 8.79 (d, J=1.6 Hz, 1H), 8.18 (d, J=1.6 Hz, 1H), 8.15 (d, J=7.8 Hz, 1H), 7.56~7.51 (m, 2H), 7.44 (d, J=8.3 Hz, 1H), 7.40 (d, J=3.7 Hz, 1H), 7.32 (t, J=7.4 Hz, 1H), 6.86~6.83 (m, 1H), 3.74 (s, 3H), 2.59 (s, 3H), 2.57 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 187.36, 186.68, 152.20, 149.60, 142.52, 142.36, 141.55, 140.12, 137.23, 135.02, 128.21, 127.93, 127.27, 127.04, 126.61, 124.57, 124.14, 122.46, 122.10, 120.48, 120.37, 109.37, 32.68, 16.17, 15.91. HRMS (ESI) calcd for C25H20S2NO2 [M+H]+: 430.0921, found 430.0924.
(9-Benzyl-9H-carbazole-1, 3-diyl)bis(phenylmethanone) (4a)[9]: White solid, 58.2 mg, 63%. 1H NMR (400 MHz, CDCl3) δ: 8.83 (d, J=1.7 Hz, 1H), 8.25 (d, J=7.8 Hz, 1H), 7.90 (d, J=1.7 Hz, 1H), 7.86~7.84 (m, 2H), 7.63~7.57 (m, 3H), 7.55~7.46 (m, 5H), 7.44~7.40 (m, 1H), 7.34~7.27 (m, 2H), 6.99~6.85 (m, 3H), 6.68 (d, J=7.2 Hz, 2H), 5.64 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 195.64, 195.30, 142.85, 139.65, 138.12, 137.03, 135.59, 132.91, 132.04, 130.35, 129.89, 129.72, 128.31, 128.23, 128.01, 127.41, 127.36, 127.25, 126.78, 125.47, 125.36, 123.26, 122.68, 121.00, 120.58, 109.82, 48.07.
(9-Propyl-9H-carbazole-1, 3-diyl)bis(phenylmethanone) (4b)[9]: Yellow oil, 43.0 mg, 51%. 1H NMR (400 MHz, CDCl3) δ: 8.75 (d, J=1.7 Hz, 1H), 8.16 (d, J=7.8 Hz, 1H), 7.99 (d, J=1.7 Hz, 2H), 7.97~7.96 (m, 1H), 7.85~7.83 (m, 2H), 7.67~7.62 (m, 1H), 7.61~7.55 (m, 2H), 7.54~7.47 (m, 5H), 7.37~7.32 (m, 1H), 4.22 (t, J=7.6 Hz, 2H), 1.67~1.57 (m, 2H), 0.71 (t, J=7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.88, 195.74, 142.09, 139.64, 138.34, 137.35, 133.79, 132.01, 130.65, 129.91, 129.56, 128.75, 128.29, 127.19, 127.08, 125.44, 124.88, 122.83, 122.45, 120.67, 120.51, 109.94, 46.60, 21.69, 11.08.
(6, 9-Dimethyl-9H-carbazole-1, 3-diyl)bis(phenylmethan-one) (4c)[9]: White solid, 38.2 mg, 47%.1H NMR (400 MHz, CDCl3) δ: 8.71 (s, 1H), 8.03 (s, 1H), 7.99~7.93 (m, 3H), 7.84 (d, J=7.0 Hz, 2H), 7.65~7.56 (m, 2H), 7.51 (d, J=4.5 Hz, 4H), 7.37 (q, J=8.3 Hz, 2H), 3.66 (s, 3H), 2.55 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.83, 195.76, 141.22, 140.90, 138.42, 137.69, 133.70, 131.95, 130.65, 130.30, 129.88, 129.66, 128.70, 128.55, 128.28, 127.12, 125.58, 124.52, 122.79, 122.24, 120.44, 109.25, 33.19, 21.35.
(6-Methoxy-9-methyl-9H-carbazole-1, 3-diyl)bis(phenyl-methanone) (4d): White solid, 43.8 mg, 52%. 1H NMR (400 MHz, CDCl3) δ: 8.72 (d, J=1.6 Hz, 1H), 8.02 (d, J=1.6 Hz, 1H), 7.99~7.95 (m, 2H), 7.86~7.82 (m, 2H), 7.66~7.56 (m, 3H), 7.51 (td, J=7.5, 4.1 Hz, 4H), 7.37 (d, J=8.9 Hz, 4H), 7.19 (dd, J=8.9, 2.5 Hz, 4H), 3.94 (s, 3H), 3.66 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.73, 154.86, 141.36, 138.41, 137.66, 137.42, 133.71, 131.96, 130.64, 129.88, 129.82, 128.70, 128.29, 126.86, 125.65, 124.51, 123.15, 122.29, 116.51, 110.41, 103.01, 56.02, 33.26. HRMS (ESI) calcd for C28H22NO3 [M+H]+: 420.1594, found 420.1589.
(6-Fluoro-9-methyl-9H-carbazole-1, 3-diyl)bis(phenyl-methanone) (4e)[9]: White solid, 36.7 mg, 45%. 1H NMR (400 MHz, CDCl3) δ: 8.68 (s, 1H), 8.06 (s, 1H), 7.98 (d, J=7.5 Hz, 2H), 7.84~7.78 (m, 3H), 7.67~7.58 (m, 2H), 7.51 (q, J=7.6 Hz, 4H), 7.40 (dd, J=8.8, 3.9 Hz, 1H), 7.34~7.26 (m, 1H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.56, 158.14 (d, J=238.4 Hz), 141.74, 138.89, 138.18, 137.51, 133.85, 132.13, 130.68, 130.17, 129.87, 128.77, 128.35, 127.37, 125.94, 124.15 (d, J=4.1 Hz), 123.25 (d, J=9.7 Hz), 122.65, 115.07 (d, J=25.6 Hz), 110.39 (d, J=9.0 Hz), 106.36 (d, J=24.2 Hz), 33.39; 19F NMR (376 MHz, CDCl3) δ: -122.42.
(7-Chloro-9-methyl-9H-carbazole-1, 3-diyl)bis(phenyl-methanone) (4f): White solid, 37.3 mg, 44%. 1H NMR (400 MHz, CDCl3) δ: 8.69 (d, J=1.6 Hz, 1H), 8.04 (t, J=4.7 Hz, 2H), 7.99~7.96 (m, 3H), 7.82 (dd, J=5.2, 3.3 Hz, 2H), 7.68~7.62 (m, 1H), 7.62~7.56 (m, 1H), 7.56~7.47 (m, 4H), 7.46 (d, J=1.6 Hz, 1H), 7.31 (dd, J=8.3, 1.7 Hz, 1H), 3.65 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 195.57, 195.52, 143.14, 141.31, 138.12, 137.43, 133.90, 133.09, 132.15, 130.67, 129.88, 129.80, 128.78, 128.34, 127.97, 125.40, 124.18, 122.63, 121.40, 121.28, 121.20, 109.86, 33.30. HRMS (ESI) calcd for C27H19ClNO2 [M+H]+: 424.1099, found 424.1093.
Supporting Information The copies of the 1H NMR, 19F NMR, and 13C NMR spectra. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn/.
-
-
[1]
(a) Zhang, F. -F. ; Gan, L. -L. ; Zhou, C. -H. Bioorg. Med. Chem. Lett. 2010, 20, 1881.
(b) Blunt, J. W. ; Copp, B. R. ; Munro, M. H. G. ; Northcote, P. T. ; Prinsep, M. R. Nat. Prod. Rep. 2003, 20, 1.
(c) Deslandes, S. ; Chassaing, S. ; Delfourne, E. Mar. Drugs 2009, 7, 754.
(d) Maneerat, W. ; Ritthiwigrom, T. ; Cheenpracha, S. ; Promgool, T. ; Yossathera, K. ; Deachathai, S. ; Phakhodee, W. ; Laphookhieo, S. J. Nat. Prod. 2012, 75, 741. -
[2]
(a) Roy, J. ; Jana, A. K. ; Mal, D. Tetrahedron 2012, 68, 6099.
(b) Knölker, H. -J. ; Reddy, K. R. Chem. Rev. 2002, 102, 4303.
(c) Schmidt, A. W. ; Reddy, K. R. ; Knölker, H. -J. Chem. Rev. 2012, 112, 3193. -
[3]
(a) Ackermann, L. ; Vicente, R. ; Kapdi, A. R. Angew. Chem. , Int. Ed. 2009, 48, 9792.
(b) Lyons, T. W. ; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(c) Liu, C. ; Zhang, H. ; Shi, W. ; Lei, A. Chem. Rev. 2011, 111, 1780.
(d) Yeung, C. S. ; Dong, V. M. Chem. Rev. 2011, 111, 1215. -
[4]
Zhao, J.; Larock, R. C. J. Org. Chem. 2006, 71, 5340. doi: 10.1021/jo060727r
-
[5]
(a) Tsang, W. C. P. ; Zheng, N. ; Buchwald, S. L. J. Am. Chem. Soc. 2005,127, 14560.
(b) Jordan-Hore, J. A. ; Johansson, C. C. ; Beck, E. M. ; Gaunt, M. J. J. Am. Chem. Soc. 2008, 130, 16184.
(c) Cho, S. H. ; Yoon, J. ; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996.
(d) Takamatsu, K. ; Hirano, K. ; Satoh, T. ; Miura, M. Org. Lett. 2014, 16, 2892. -
[6]
(a) Yamashita, M. ; Horiguchi, H. ; Hirano, K. ; Satoh, T. ; Miura, M. J. Org. Chem. 2009, 74, 7481.
(b) Jia, J. ; Shi, J. ; Zhou, J. ; Liu, X. ; Song, Y. ; Xu, H. E. ; Yi, W. Chem. Commun. 2015, 51, 2925. -
[7]
(a) Ozaki, K. ; Zhang, H. ; Ito, H. ; Lei, A. ; Itami, K. Chem. Sci. 2013, 4, 3416.
(b) Verma, A. K. ; Danodia, A. K. ; Saunthwal, R. K. ; Patel, M. ; Choudhary, D. Org. Lett. 2015, 17, 3658.
(c) Laha, J. K. ; Dayal, N. Org. Lett 2015, 17, 4742.
(d) Chen, S. ; Li, Y. ; Ni, P. ; Huang, H. ; Deng, G. J. Org. Lett 2016. -
[8]
Grimster, N. P.; Gauntlett, C.; Godfrey, C. R.; Gaunt, M. J. Angew. Chem., Int. Ed. 2005, 44, 3125. doi: 10.1002/(ISSN)1521-3773
-
[9]
Guo, T.; Jiang, Q.; Huang, F.; Chen, J.; Yu, Z. Org. Chem. Front. 2014, 1, 707. doi: 10.1039/C4QO00122B
-
[10]
(a) Muzart, J. Eur. J. Org. Chem. 2010, 3779.
(b) Newhouse, T. ; Turlik, A. ; Chen, Y. Synlett 2016, 27, 331. -
[11]
(a) Nicolaou, K. C. ; Gray, D. L. F. ; Montagnon, T. ; Harrison, S. T. Angew. Chem. , Int. Ed. 2002, 41, 996.
(b) Nicolaou, K. C. ; Montagnon, T. ; Baran, P. S. Angew. Chem. , Int. Ed. 2002, 41, 993.
(c) Nicolaou, K. C. ; Montagnon, T. ; Baran, P. S. ; Zhong, Y. L. J. Am. Chem. Soc. 2002, 124, 2245.
(d) Uyanik, M. ; Akakura, M. ; Ishihara, K. J. Am. Chem. Soc. 2009, 131, 251. (e) Nicolaou, K. C. ; Zhong, Y. L. ; Baran, P. S. J. Am. Chem. Soc. 2000, 122, 7596. -
[12]
(a) Bhattacharya, A. ; DiMichele, L. M. ; Dolling, U. H. ; Douglas, A. W. ; Grabowski, E. J. J. J. Am. Chem. Soc. 1988, 110, 3318.
(b) Walker, D. ; Hiebert, J. D. Chem. Rev. 1967, 67, 153. -
[13]
(a) Diao, T. ; Stahl, S. S. J. Am. Chem. Soc. 2011, 133, 14566.
(b) Gao, W. M. ; He, Z. Q. ; Qian, Y. ; Zhao, J. ; Huang, Y. Chem. Sci. 2012, 3, 883.
(c) Diao, T. ; Wadzinski, T. J. ; Stahl, S. S. Chem. Sci. 2012, 3, 887.
(d) Diao, T. ; Pun, D. ; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 8205.
(e) Bigi, M. A. ; White, M. C. J. Am. Chem. Soc. 2013, 135, 7831. -
[14]
(a) Zhang, M. ; Zhang, Y. ; Jie, X. ; Zhao, H. ; Li, G. ; Su, W. Org. Chem. Front. 2014, 1, 843.
(b) Wei, Y. ; Hu, P. ; Zhang, M. ; Su, W. Chem. Rev. 2017. -
[15]
(a) Zhang, M. ; Zhou, J. ; Kan, J. ; Wang, M. ; Su, W. ; Hong, M. Chem. Commun. 2010, 46, 5455.
(b) Zhou, J. ; Wu, G. ; Zhang, M. ; Jie, X. ; Su, W. Chem. -Eur. J. 2012, 18, 8032.
(c) Zhang, M. ; Hu, P. ; Zhou, J. ; Wu, G. ; Huang, S. ; Su, W. Org. Lett. 2013, 15, 1718.
(d) Shang, Y. ; Jie, X. ; Zhou, J. ; Hu, P. ; Huang, S. ; Su, W. Angew. Chem. Int. Ed. 2013, 52, 1299. -
[16]
Jie, X.; Shang, Y.; Zhang, X.; Su, W. J. Am. Chem. Soc. 2016, 138, 5623. doi: 10.1021/jacs.6b01337
-
[17]
Xiao, B.; Li, Y. M.; Liu, Z. J.; Yang, H. Y.; Fu, Y. Chem. Commun. 2012, 48, 4854. doi: 10.1039/c2cc31737k
-
[18]
Klare, H. F.; Oestreich, M.; Ito, J.; Nishiyama, H.; Ohki, Y.; Tatsumi, K. J. Am. Chem. Soc. 2011, 133, 3312. doi: 10.1021/ja111483r
-
[19]
Taylor, J. E.; Jones, M. D.; Williams, J. M. J.; Bull, S. D. Org. Lett. 2010, 12, 5740. doi: 10.1021/ol1025348
-
[20]
Qi, T.; Qiu, W.; Liu, Y.; Zhang, H.; Gao, X.; Liu, Y.; Lu, K.; Du, C.; Yu, G.; Zhu, D. J. Org. Chem. 2008, 73, 4638. doi: 10.1021/jo800622y
-
[1]
-
Table 1. Optimization studies of reaction conditionsa

Entry Ag2CO3/ equiv. Ligand Base TEMPO/ equiv. Yieldb /% of3a 1 3.0 PCy3 LiOAc 0.4 77 (76) 2c 3.0 PCy3 LiOAc 0.4 0 3 0 PCy3 LiOAc 0.4 34 4 3.0 PCy3 LiOAc 0.0 26 5 3.0 LiOAc 0.4 Trace 6 3.0 PCy3 0.4 28 7 1.0 PCy3 LiOAc 0.4 45 8 2.0 PCy3 LiOAc 0.4 58 9 3.0 PPh3 LiOAc 0.4 Trace 10 3.0 Xphos LiOAc 0.4 Trace 11 3.0 Davephos LiOAc 0.4 Trace 12 3.0 PCy3 NaOAc 0.4 15 13 3.0 PCy3 KOAc 0.4 16 14 3.0 PCy3 Na2CO3 0.4 10 15 3.0 PCy3 K2CO3 0.4 Trace 16d 3.0 PCy3 LiOAc 0.4 50 17e 3.0 PCy3 LiOAc 0.4 25 aReaction conditons: 1a (0.2 mmol), 2a (10 equiv.), Pd(OAc)2 (10 mol%), ligand(25 mol%), Ag2CO3 (3.0 equiv.), base (1.0 equiv.), TEMPO (0.4 equiv.), 101 kPa O2, DME, 110 ℃, 24 h.; bNMR yield using CH2Br2 as internal standard (values in parentheses refers to the isolated yield); cin absence of Pd(TFA)2.; d the reaction was conducted under air.; ethe reaction was conducted under N2 atmosphere. Table 2. Scope of propiophenonesa

Table 3. Scope of indoles

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 9
- 文章访问数: 2291
- HTML全文浏览量: 125



 下载:
下载:

 下载:
下载:

