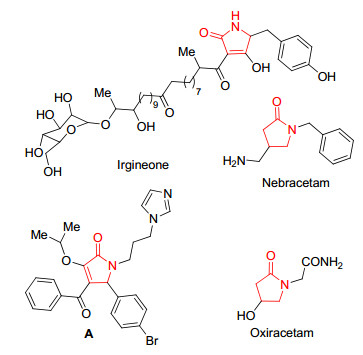
 图1
具有生物活性的吡咯酮化合物
Figure1.
Pyrrolone compounds with biologically activities
图1
具有生物活性的吡咯酮化合物
Figure1.
Pyrrolone compounds with biologically activities

Citation: Zhao Yucheng, Xiao Qiang, Wang Baoqu, Lin Jun, Yan Shengjiao. Synthesis of Iminopyrrolone Compounds[J]. Chinese Journal of Organic Chemistry, 2017, 37(10): 2690-2696. doi: 10.6023/cjoc201705004

亚胺基吡咯酮类化合物的合成
English
Synthesis of Iminopyrrolone Compounds
-
Key words:
- iminopyrrolone
- / nitro-enamine
- / synthesis
-
吡咯酮是一类重要的杂环化合物, 可作为合成重要天然产物、药物及有机配体的中间体[1].很多天然产物和生物碱都具有吡咯酮结构, 如对多种真菌具有抑制活性的天然产物Irgineone[2]具有吡咯酮骨架结构.吡咯酮衍生物具有广谱的生物活性, 如抗肿瘤[3]、抗真菌[4]、抗艾滋[5]、镇静[6]、除草[7]、杀虫[8]、治疗老年痴呆[9]等多种活性[10].如Zhang课题组[11]合成以p53-MDM2蛋白为靶点的抗肿瘤抑制剂(图 1, A).目前已有多种吡咯酮药物批准用于临床使用, 如具有镇静功能的抗癫痫药物奈拉西坦(Nebracetam), 治疗脑损伤的奥拉西坦(Oxiracetam)等(图 1)[12].由于吡咯酮类化合物广谱的生物活性, 该类化合物多年来一直备受有机化学和药物学家广泛关注, 到目前为止已有大量文献报道合成该类化合物的方法[13, 14].但文献报道的吡咯酮类化合物多属于常见的结构, 而一些特殊的吡咯酮类化合物如亚胺类的吡咯酮类化合物由于亚胺的不稳定性, 导致研究合成该类化合物的方法鲜见报道.所以研究合成亚胺类吡咯酮化合物对研究开发吡咯酮类药物具有十分重要的意义.
1, 1-烯二胺类化合物包括链状1, 1-烯二胺(1, 1-ene-diamines)和环状1, 1-烯二胺[cyclic 1, 1-enediamines或heterocyclic ketene aminas (HKAs)] (图 2).环状1, 1-烯二胺类化合物是一种多功能合成砌块, 因其结构中具有吸电子基和供电性胺基, 导致碳-碳双键高度极化, 致使α-碳电子云密度增大, 使得其结构中具有三个亲核位点, 可以与多种亲电试剂发生选择性反应.如烷基化反应[15]、酰化反应[16]、胺化反应[17]、烷硫基化[18]等反应.环状1, 1-烯二胺通常用于构建常规方法难以获得的杂环化合物[19, 20]和稠杂环化合物[21], 而链状1, 1-烯二胺类化合物具有与环状1, 1-烯二胺相似的结构, 应该具有相似的化学性质或细微的差别.但到目前为止, 该类化合物并未受到化学及药物学家关注[22~25].因此, 探索以链状1, 1-烯二胺为砌块合成具有潜在生物活性的杂环化合物具有重要的学术及应用价值.
以1, 1-烯二胺化合物1和廉价易得的顺丁烯二酸酐(2)为原料, 三乙胺为碱, 1, 4-二氧六环为溶剂, 回流条件下, 通过迈克尔加成、环化、脱羧、脱硝基反应构建结构新颖的亚胺类吡咯酮化合物.该方法具有原料易得、合成路线简洁、操作简便等优点.合成路线见Eq. 1.
1 结果与讨论
1.1 反应条件筛选
我们以1a与2为模板反应, 分别对反应碱、溶剂、反应时间、温度等进行条件筛选与优化, 结果见表 1.

Entry Catalyst Solvent T/℃ Time/h Yieldb /% 1 — 1, 4-Dioxane r.t. 8 — 2 — 1, 4-Dioxane Reflux 8 — 3 Et3Nc 1, 4-Dioxane Reflux 2 56 4 Piperidinec 1, 4-Dioxane Reflux 2 12 5 Cs2CO3 d 1, 4-Dioxane Reflux 2 5 6 t-BuOKd 1, 4-Dioxane Reflux 2 — 7 AcOHc 1, 4-Dioxane Reflux 2 — 8 Et3Nc EtOH Reflux 2 — 9 Et3Nc THF Reflux 2 — 10 Et3Nc MeCN Reflux 2 — 11 Et3Nc DMF 100 2 23 12 Et3Nc Toluene Reflux 2 41 13 Et3N c 1, 4-Dioxane Reflux 4 68 14 Et3Nc 1, 4-Dioxane Reflux 8 67 aThe reaction was performed with 1a (1.0 mmol), 2 (2.0 mmol).bIsolated yields based on 1a.c The catalyst 0.05 mmol.d The catalyst 2.0 mmol. 首先, 我们将1a与2以1:2的投料比置于1, 4-二氧六环中, 室温条件下搅拌8 h后, 薄层色谱(TLC)检测表明没有任何新点产生(表 1, Entry1).在同样条件下, 把室温改为加热回流, 回流8 h后, TLC检测表明没有明显新点产生.之后以三乙胺(0.05 Equiv.)为催化剂, 在1, 4-二氧六环中回流2 h, 结果以56%的产率得化合物3a (Table 1, Entry 3).在此基础上, 我们筛选了哌啶、碳酸铯、叔丁醇钾和醋酸三种碱、一种酸.结果表明该反应在有机弱碱, 如哌啶的催化下可以获得少量的目标产物, 当增加有机碱的碱性, 如使用叔丁醇钾后, 原料顺丁烯二酸酐很快消失; 而使用无机碱和有机酸作为催化剂时, 并不能得到目标产物(Table 1, Entries 5~7).然后, 我们以三乙胺为催化剂, 筛选了乙醇、四氢呋喃、乙腈、N, N-二甲基甲酰胺(DMF)和甲苯五种溶剂, 发现在低沸点的溶剂中回流无法得到目标产物(Table 1, Entries 8~10);在沸点超过100 ℃的溶剂中便可获得目标产物(表 1, Entries 11~12).最后, 我们以三乙胺作为催化剂, 以1, 4-二氧六环为溶剂, 对反应时间进行筛选, 发现反应时间为4 h时, 产率明显提高; 继续增加反应时间, 产率却没有明显的提高(表 1, Entry 3 vs. 13~14).最终确定最佳反应条件为: n(1a):n(2)=1:2, 以三乙胺(0.05 equiv.)为催化剂, 在1, 4-二氧六环中回流4 h.
1.2 反应底物对产率影响
在最佳条件基础上, 对该反应的普适性进行探索(表 2).选择苯环上为供电子基甲基、甲氧基和吸电子基氟、氯和多氟及不同链长(n=1, 2) 的反应底物1进行测试.研究结果表明, 苯环上具有吸电子基团(F、Cl、CF3)底物比供电子基团(Me、OMe)取代的底物具有更高的产率(3a~3f); 同时, 苯环上吸电子基从一个增加为两个时, 产物产率明显提高(3b vs. 3g~3h), 苄基被α-甲基苄基取代后, 产率有明显下降(3a vs. 3j); 苯环的邻位为吸电子基团时, 产物的产率也会提高(3g vs. 3h; 3k vs. 3l).此外, 当增加烯胺和苯环之间的链长时, 产物的产率也会提高(3b vs. 3k~3l).
1.3 反应机理
我们以化合物3a为例, 我们推测反应的可能机理:首先1a对顺丁烯二酸酐2进行迈克尔加成[27]反应得中间体4a, 中间体4a在碱的作用下脱去一分子亚硝酸[28]得中间体5a, 中间体5a经分子内环化加成反应形成中间体6a, 中间体6a开环得中间体7a, 中间体7a在加热下脱羧[29]得到目标化合物3a.反应机理如下Scheme 1.
1.4 化合物晶体结构
化合物3c的晶体数据及结构修正见表 3.分子结构见图 3.单晶cif文件寄存于剑桥晶体数据中心(CCDC号为1546965).
Empirical formula C19H16Cl2N2O Formula weight 359.24 Temperature 293.15 K Wavelength 0.71073 Å Crystal system, space group Triclinic, P-1 Unit cell dimensions a=7.5752(12) Å b=10.7338(17) Å c=12.270(3) Å α=112.537(3)° β=105.009(3)° γ=95.266(2)° Volume 869.4(3) Å3 Z, Calculated density 2, 1.372 Mg/m3 F(000) 372.0 Crystal size 0.1 mm×0.28 mm×0.42 mm Theta range for data collection 3.794° to 50.294° Limiting indices -9≤h≤9 -12≤k≤12, -14≤l≤14 Reflection collected 6847 independent reflections 3084 [Rint=0.0199, Rσ=0.0261] Data/restraints/parameters 3084/0/227 Absorption correction Semi-empirical from equivalents Refinement method Full-matrix least-squares on F2 Goodness-of-fit on F2 1.069 Final R indices [I>2σ(I)] R1=0.0349, wR2=0.0898 Final R indexes [all data] R1=0.0407, wR2=0.0951 Largest diff. peak and hole 0.18/-0.32 A-3 2 结论
本文成功建立了一种一锅法简洁、高效合成具有潜在生物活性的亚胺基吡咯酮类化合物的方法.该方法以1, 1-烯二胺类化合物1与顺丁烯二酸酐(2)在1, 4-二氧六环溶剂中, 三乙胺为碱的条件下加热回流反应, 通过迈克尔加成、缩合、脱羧和脱二氧化氮快速构建结构新颖的亚氨基吡咯酮类化合物3.该类吡咯酮类化合物的一锅法制备为今后进行广泛的生物活性研究奠定了一定的基础.该方法不仅具有合成路线简单, 原料易得等优点, 更重要的是开拓了链状1, 1-烯二胺的在杂环化合物合成中的应用.
3 实验部分
3.1 仪器和试剂
控温型电磁搅拌器; 傅里叶红外光谱仪(Thermo Nicolet Avatar 360型); 高分辨质谱仪(Agilent CL/Msd TOF); 核磁共振仪Bruck DRX300 (1H: 300 MHz, 13C:75 MHz)或DRX500 (1H: 500 MHz, 13C: 125 MHz); XT-4A控温型显微熔点测定仪.试剂为分析纯或化学纯(无水硫酸钠干燥处理), GF254高效薄层层析板及柱层析硅胶(200~300目, 青岛海洋化工厂).反应原料1按文献[26]制备.
3.2 实验方法
称取1 mmol硝基烯胺1和2 mmol顺丁烯二酸酐(2), 加入到25 mL圆底烧瓶中, 再加入10 mL 1, 4-二氧六环搅拌溶解, 搅拌下, 加入0.05 mmol三乙胺作催化剂, 加热回流4 h, TLC监测反应完全后, 停止加热.将反应液冷却至室温, 加入10 mL水搅拌5 min后, 用乙酸乙酯(10 mL×3) 萃取, 乙酸乙酯层用无水硫酸钠干燥后, 通过柱层析色谱分离得到目标产物3, 产率为68%~90%.所有的化合物都经过核磁共振、红外、高分辨质谱验证.
1-苄基-5-(苄基亚氨基)-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3a):黄色油状液体, 产率68%, E:Z=51:49. 1H NMR (500 MHz, CDCl3) δ: 2.05 (s, 1.38H, CH3, Z), 2.06 (s, 1.78H, CH3, E), 4.81 (s, 2H, CH2), 4.85 (s, 2H, CH2), 6.82 (s, 0.49H, =CH, Z), 6.82 (s, 0.51H, =CH, E), 7.20~7.29 (m, 8H, ArH), 7.30~7.37 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) δ: 11.3, 42.1, 54.4, 118.7, 126.9, 127.2, 127.3, 128.3, 128.4, 137.8, 139.8, 142.3, 155.7, 171.1; IR (KBr) ν: 3432, 3086, 3027, 2925, 1907, 1660, 1604, 1584, 1453, 1404, 1352, 1296, 1149, 1071, 965, 871, 726, 697, 600 cm-1; HRMS (TOF ES+) calcd for C19H19N2O 291.1492, found 291.1490.
1-(4-氟苄基)-5-[(4-氟苄基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3b):黄色油状液体, 产率79%, E: Z=51:49. 1H NMR (300 MHz, CDCl3) δ: 2.06 (s, 1.53H, CH3, E), 2.07 (1.46H, CH3, Z), 4.76 (s, 2H, CH2), 4.80 (s, 2H, CH2), 6.82 (s, 0.51H, =CH, E), 6.82 (s, 0.51H, =CH, Z), 6.91~7.03 (m, 4H, ArH), 7.15~7.20 (m, 2H, ArH), 7.31~7.35 (m, 2H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.3, 42.3, 53.7, 115.0, 115.1, 115.3, 115.4, 118.7, 128.8, 128.9, 130.2, 130.3, 133.6, 135.4, 142.6, 155.6, 160.2, 160.5, 163.5, 163.7, 171.1; IR (KBr) ν: 3432, 3060, 2938, 1721, 1650, 1601, 1434, 1409, 1350, 1214, 1152, 1087, 960, 857, 841, 763, 670, 588 cm-1; HRMS (TOF ES+) calcd for C19H17F2N2O 327.1303, found 327.1304.
1-(4-氯苄基)-5-[(4-氯苄基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3c):黄色油状液体, 产率77%, E: Z=55:45. 1H NMR (500 MHz, CDCl3) δ: 2.06 (s, 1.46H, CH3, Z), 2.06 (s, 1.62H, CH3, E), 4.75 (s, 2H, CH2), 4.79 (s, 2H, CH2), 6.80 (s, 0.45H, =CH, Z), 6.81 (s, 0.54H, =CH, E), 7.12~7.22 (m, 2H, ArH), 7.24~7.29 (m, 6H, ArH); 13C NMR (125 MHz, CDCl3) δ: 11.3, 41.4, 53.6, 118.8, 128.5, 128.6, 128.6, 129.9, 132.7, 133.1, 133.2, 138.2, 142.6, 155.7, 170.1; IR (KBr) ν: 3422, 2920, 1716, 1656, 1595, 1488, 1434, 1403, 1345, 1332, 1290, 1149, 1109, 1088, 1015, 960, 851, 833, 807, 800, 756, 646, 581 cm-1; HRMS (TOF ES+) calcd for C19H17Cl2N2O, 359.0712, found 359.0710.
3-甲基-1-[4-(三氟甲基)苄基]-5-[(4-三氟甲基苄基)亚氨基]-1, 5-二氢-2H-吡咯-2-酮(3d):黄色油状液体, 产率71%, E:Z=56:44. 1H NMR (500 MHz, CDCl3) δ: 2.09 (s, 1.63H, CH3, E), 2.09 (s, 1.37H, CH3, E), 4.84 (s, 2H, CH2), 4.90 (s, 2H, CH2), 6.84 (s, 0.55H, =CH, E), 6.84 (s, 0.44H, =CH, Z), 7.29~7.30 (m, 2H, ArH), 7.44~7.45 (m, 2H, ArH), 7.53~7.56 (m, 4H, ArH); 13C NMR (125 MHz, CDCl3) δ: 10.3, 40.7, 52.8, 117.8, 124.4, 124.4, 124.4, 126.5, 127.5, 140.6, 141.8, 142.6, 154.8, 169.9; IR (KBr) ν: 3441, 2922, 1720, 1664, 1620, 1418, 1332, 1158, 1111, 1068, 1017, 970, 848, 821, 750, 723, 679 cm-1; HRMS (TOF ES+) calcd for C21H17N2O 427.124, found 427.1238.
3-甲基-1-(4-甲基苄基)-5-[(4-甲基苄基)亚氨基]-1, 5-二氢-2H-吡咯-2-酮(3e):黄色油状液体, 产率69%, E:Z=51:49. 1H NMR (300 MHz, CDCl3) δ: 2.03 (s, 1.52H, CH3, E), 2.04 (s, 1.49H, CH3, Z), 2.30 (s, 3H, CH3), 2.33 (s, 3H, CH3), 4.77~4.80 (m, 4H, CH2), 6.80 (s, 0.51H, =CH, E), 6.80 (s, 0.49H, =CH, Z), 7.06~7.12 (m, 6H, ArH), 7.24~7.28 (m, 2H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.3, 21.2, 41.7, 54.2, 118.7, 127.3, 128.5, 129.0, 129.1, 134.9, 136.5, 136.8, 142.3, 155.7, 171.2; IR (KBr) ν: 3431, 2922, 1716, 1657, 1515, 1432, 1402, 1347, 1306, 1263, 1151, 1115, 1040, 959, 846, 801, 862, 626 cm-1; HRMS (TOF ES+) calcd for C21H23N2O 319.1805, found 319.18.
1-(4-甲氧基苄基)-5-[(4-甲氧基苄基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3f):黄色油状液体, 产率77%, E:Z=51:49. 1H NMR (300 MHz, CDCl3) δ: 2.02 (s, 1.5H, CH3, E), 2.02 (s, 1.46H, CH3, Z), 3.73 (s, 3H, CH3), 3.77 (s, 3H, CH3), 4.73~4.76 (m, 4H, CH2), 6.76~6.80 (m, 2H, ArH), 6.79 (s, 1H, =CH), 6.83~6.86 (m, 2H, ArH), 7.13~7.16 (m, 2H, ArH), 7.29~7.32 (m, 2H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.3, 41.4, 53.8, 55.2, 55.3, 113.7, 113.9, 118.8, 128.5, 130.0, 130.1, 131.9, 142.2, 155.6, 158.8, 171.2; IR (KBr) ν: 3433, 2999, 2934, 2835, 1717, 1657, 1612, 1512, 1462, 1432, 1402, 1347, 1301, 1248, 1176, 1150, 1033, 961, 818, 807, 765, 623, 589, 540 cm-1; HRMS (TOF ES+) calcd for C19H23N2O3 351.1703, found 351.17.
1-(2, 4-二氟苄基)-5-[(2, 4-二氟苄基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3g):黄色油状液体, 产率88%, E:Z=52:48. 1H NMR (300 MHz, CDCl3) δ: 2.09 (s, 1.47H, CH3, Z), 2.09 (s, 1.56H, CH3, E), 4.75 (s, 2H, CH2), 4.86 (s, 2H, CH2), 6.73~6.84 (m, 4H, ArH), 6.89 (s, 0.52H, =CH, E), 6.89 (s, 0.48H, =CH, E), 7.16~7.21 (m, 2H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.3, 35.3 (d, J=4.5 Hz), 47.3, 103.5 (t, J=25.5 Hz), 103.7 (t, J=25.5 Hz), 111.0 (d, J=18.0 Hz), 111.1 (d, J=21.0 Hz), 119.0, 120.5 (d, J=18.0 Hz), 120.5 (d, J=11.3 Hz), 122.6 (d, J=18.0 Hz), 130.1 (t, J=9.0 Hz), 130.8 (t, J=8.0 Hz), 142.6, 155.9, 159.9 (dd, J=223.5, 22.5 Hz), 160.5 (dd, J=248.3, 22.5 Hz), 160.5, 160.8 (dd, J=259.5, 25.5 Hz), 170.9; IR (KBr) ν: 3424, 3090, 2952, 2885, 1722, 1664, 1616, 1505, 1437, 1405, 1387, 1353, 1277, 1168, 1140, 1107, 995, 973, 963, 859, 851, 818, 787 cm-1; HRMS (TOF ES+) calcd for C19H15F4N2O 363.1115, found 363.1114.
1-(3, 4-二氟苄基)-5-[(3, 4-二氟苄基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3h):黄色油状液体, 产率84%, E:Z=53:47. 1H NMR (300 MHz, CDCl3) δ: 2.08 (s, 1.52H, CH3, E), 2.08 (s, 1.49H, CH3, Z), 4.74 (s, 2H, CH2), 4.77 (s, 2H, CH2), 6.82 (s, 0.51H, =CH, E), 6.82 (s, 0.46H, =CH, Z), 6.96~7.20 (m, 6H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.3, 41.1, 53.2, 116.2 (d, J=17.3 Hz), 117.0, 117.2 (d, J=20.3 Hz), 117.4 (d, J=27.0 Hz), 118.8, 123.0, 124.5, 134.5, 136.6, 142.9, 148.6, 155.7, 170.9; IR (KBr) ν: 3381, 3069, 2925, 1725, 1662, 1610, 1519, 1437, 1352, 1284, 1209, 1137, 1116, 972, 841, 779, 762, 575 cm-1; HRMS (TOF ES+) calcd for C19H15F4N2O 363.1115, found, 363.1115.
3-甲基-1-(1-苯基乙基)-5-[(1-苯基乙基)亚氨基]-1, 5-二氢-2H-吡咯-2-酮(3i):黄色油状液体, 产率68%, E: Z=100:0. 1H NMR (300 MHz, CDCl3) δ: 1.59 (d, J=6.6 Hz, 3H, CH3), 2.04 (d, J=7.2 Hz, 3H, CH3), 2.09 (s, 3H, CH3), 4.95~5.01 (m, 1H, CH), 5.82~5.89 (m, 1H, CH), 6.87 (s, 1H, =CH), 7.32~7.50 (m, 8H, ArH), 7.53~7.61 (m, 2H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.3, 17.9, 26.7, 49.6, 59.7, 118.6, 126.3, 126.9, 127.1, 127.6, 128.2, 128.6, 141.9, 142.1, 146.0, 153.8, 171.2; IR (KBr) ν: 3424, 2932, 1718, 1656, 1602, 1509, 1437, 1407, 1364, 1221, 1157, 1097, 825, 538 cm-1; HRMS (TOF ES+) calcd for C21H23N2O 319.1805, found 319.1803.
3-甲基-1-苯乙基-5-(苯乙基亚氨基)-1, 5-二氢-2H-吡咯-2-酮(3j):黄色油状液体, 产率89%, E:Z=54:46. 1H NMR (300 MHz, CDCl3) δ: 1.89 (s, 1.56H, CH3, E), 1.90 (s, 1.45H, CH3, Z), 2.81~2.91 (m, 4H, CH2), 3.75~3.82 (m, 4H, CH2), 6.42 (s, 0.54H, =CH, E), 6.42 (s, 0.46H, =CH, Z), 7.13~7.18 (m, 6H, ArH), 7.21~7.23 (m, 4H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.1, 34.7, 38.0, 39.8, 52.6, 118.4, 126.3, 128.3, 128.4, 128.4, 129.0, 129.1, 139.0, 139.7, 141.4, 155.4, 171.2; IR (KBr) ν: 3434, 3061, 3026, 2930, 2862, 1720, 1657, 1603, 1496, 1453, 1407, 1364, 1146, 1081, 1030, 984, 841, 750, 700, 559 cm-1; HRMS (TOF ES+) calcd for C21H23N2O 319.1805, found 319.1803.
1-(2-氟苯乙基)-5-[(2-氟苯乙基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3k):黄色油状液体, 产率90%, E/Z=52:48. 1H NMR (400 MHz, CDCl3) δ: 1.94 (s, 1.65H, CH3, E), 1.95 (s, 1.64H, CH3, Z), 2.81~2.85 (m, 2H, CH2), 2.88~2.91 (m, 2H, CH2), 3.76~3.81 (m, 4H, CH2), 6.48 (s, 0.48H, =CH, Z), 6.48 (s, 0.51H, =CH, E), 6.92~6.97 (m, 4H, ArH), 7.11~7.15 (m, 4H, ArH); 13C NMR (100 MHz, CDCl3) δ: 11.0, 33.8, 37.1, 39.7, 52.5, 115.1 (d, J=20.9 Hz), 115.1 (d, J=21.1 Hz), 118.2, 130.3, 130.3, 134.9, (d, J=81.3 Hz), 135.0 (d, J=81.2 Hz), 141.6, 155.3, 161.5 (d, J=242.8 Hz), 161.6 (d, J=242.5 Hz), 171.2; IR (KBr) ν: 3441, 2936, 2870, 1729, 1657, 1602, 1552, 1510, 1438, 1405, 1384, 1223, 1212, 1144, 1110, 1034, 982, 831, 770, 759, 578 cm-1; HRMS (TOF ES+) calcd for C21H21F2N2O 355.1616, found 355.1619.
1-(4-氟苯乙基)-5-[(4-氟苯乙基)亚氨基]-3-甲基-1, 5-二氢-2H-吡咯-2-酮(3l):黄色油状液体, 产率86%, E: Z=52:48. 1H NMR (300 MHz, CDCl3) δ: 1.93 (s, 1.42H, CH3, Z), 1.94 (s, 1.67H, CH3, E), 2.90~2.94 (m, 4H, CH2), 3.75~3.80 (m, 2H, CH2), 3.83~3.88 (m, 2H, CH2), 6.53 (s, 0.48H, =CH, Z), 6.54 (s, 0.51H, =CH, E), 6.96~7.09 (m, 4H, ArH), 7.11~7.21 (m, 4H, ArH); 13C NMR (75 MHz, CDCl3) δ: 11.1, 28.0, 31.5, 38.3, 50.9, 115.1 (d, J=29.0 Hz), 115.2 (d, J=29.0 Hz), 118.3, 123.9 (d, J=3.0 Hz), 124.0 (d, J=3.8 Hz), 125.9 (d, J=16.5 Hz), 126.5 (d, J=15.8 Hz), 128.1, 128.2, 131.1 (d, J=4.5 Hz), 131.6 (d, J=4.5 Hz), 141.5, 155.5, 161.3 (d, J=243.0 Hz), 161.5 (d, J=244.5 Hz), 171.1; IR (KBr) ν: 3428, 3063, 2935, 2867, 1722, 1658, 1585, 1493, 1438, 1407, 1366, 1229, 1180, 1148, 1105, 1038, 985, 844, 756, 560 cm-1; HRMS (TOF ES+) calcd for C21H21F2N2O 355.1616, found 355.1615.
辅助材料(Supporting Information)化合物的1H NMR和13C NMR谱图.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
(a) Cramer, N.; Buchweiz, M.; Laschat, S.; Frey, W.; Baro, A.; Mathieu, D.; Richter, C.; Schwalbe, H. Chem.-Eur. J. 2006, 12, 2448.
(b) Cramer, N.; Laschat, S.; Baro, A.; Sehwalbe, H.; Richter, C. Angew. Chem., lnt. Ed. 2005, 44, 820.
(c) Yoshinari, T.; Ohmori, K.; Schrems, M. G.; Pfaltz, A.; Suzuki, K. Angew. Chem., lnt. Ed. 2009, 48, 1. -
[2]
Ondeyka, J.; Harris, G.; Zink, D.; Basilio, A.; Vicente, F.; Bills, G.; Collado, J.; Singh, S. B. J. Nat. Prod. 2009, 72, 136. doi: 10.1021/np800511r
-
[3]
(a) Lin, Z. J.; Lu, Z. Y.; Zhu, T. J.; Fang, Y. C.; Gu, Q. Q.; Zhu, W. M. Chem. Pharm. Bull. 2008, 56, 217.
(b) Sugie, Y.; Dekker, K. A.; Inagaki, T.; Kim, Y. J.; Sakakibara, J. A.; Kojima, Y. J. Antibiot. 2002, 55, 19.
(c) Heiiwig, V.; Grothe, T.; Bartschmid, A. M.; Endermann, R.; Geschke, F.; Henkei, T.; Stadler, M. J. Antibiot. 2002, 55, 881. -
[4]
(a) Wang, C. Y.; Wang, B. G.; Wiyowidagdo, S.; Wray, V.; Soest, R. V.; Steube, K. G.; Guan, H. S.; Proksch, P.; Ebel, R. J. Nat. Prod. 2003, 66, 51.
(b) Vangun, H. V. K.; Hertweck, C. Org. Biomol. Chem. 2007, 5, 1702.
(c) Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.; Ghisalberti, E. L. J. Nat. Prod. 2009, 72, 2032.
(d) Li, j.; Liu, S.; Niu, S.; Zhuang, W.; Che, Y. J. Nat. Prod. 2009, 72, 2184. -
[5]
Regueiro-Ren, A.; Xue, Q.-M.; Swidorski, J. J.; Gong, Y.-F.; Mathew, M.; Parker, D. D.; Yang, Z.; Eggers, B.; D'Arienzo, C.; Sun, Y.-N.; Malinowski, J.; Gao, Q.; Wu, D.-D.; Langley, D. R.; Colonno, R. J.; Chien, C.; Grasela, D. M.; Zheng, M.; Lin, P.-F.; Meanwell, N. A.; Kadow, J. F. J. Med. Chem. 2013, 56, 1656. doi: 10.1021/jm3016377
-
[6]
(a) Takeo, S.; Hayashi, H.; Miyake, K.; Takagi, K.; Tadokoro, M.; Takagi, N. Br. J. Pharmacol. 1997, 121, 477.
(b) Oyaizu, M.; Narahashi, T. Brain Res. 1999, 822, 72. -
[7]
(a) Royles, B. J. L. Chem. Rev. 1995, 95, 1981.
(b) Schobert, R.; Schlenk, A. Bioorg. Med. Chem. 2008, 16, 4203. -
[8]
Graupner, P.; Carr, A.; Clancy, E.; Gilbert, J.; Bailey, K. L.; Derby, J. A.; Gerwick, B. C. J. Nat. Prod. 2003, 66, 1558. doi: 10.1021/np030193e
-
[9]
Hardy, J.; Allsop, D. Trends Pharmacol. Sci. 1991, 12, 383. doi: 10.1016/0165-6147(91)90609-V
-
[10]
(a) Ramana, C. V.; Mondal, M. A.; Paranic, V. G.; Gurjar, M. K. Tetrahedron Lett. 2006, 47, 4061.
(b) Marquardt, U.; Schmid, D.; Jung, G. Synlett 2000, 1131. -
[11]
Zhuang, C.; Miao, Z.; Zhu, L.; Dong, G.; Guo, Z.; Wang, S.; Zhang, Y.; Wu, Y.; Yao, J.; Sheng C.; Zhang, W. J. Med. Chem. 2012, 22, 9630.
-
[12]
Shorvon, S. Lancet 2001, 358, 1885. doi: 10.1016/S0140-6736(01)06890-8
-
[13]
(a) Majumdar, P. ; Pati, A. ; Patra, M. ; Behera, R. K. ; Behera, A. K. Chem. Rev. 2014, 114, 2942.
(b) Eftekhari, B. ; Zirak, M. Chem. Rev. 2015, 115, 151.
(c) Eftekhari, B. ; Zirak, M. ; Akbari, A. Chem. Rev. 2013, 113, 2958.
(d) Gulevich, A. V. ; Dudnik, A. S. ; Chernyak, N. ; Gevorgyan, V. Chem. Rev. 2013, 113, 3084.
(e) Wei, L. ; Yang, X. ; Yuan, J. ; Hu, H. ; Luo, G. Chin. J. Org. Chem. 2012, 32, 2339(in Chinese).
(韦丽, 杨晓丽, 袁吉文, 胡宏纹, 陆国元, 有机化学, 2012, 32, 2339. )
(f) Tian, S. ; Hao, Y. Chin. J. Org. Chem. 2013, 33, 2232(in Chinese).
(田拴宝, 郝永兵, 有机化学, 2013, 33, 2232. ) -
[14]
(a) Candeias, N. R. ; Branco, L. C. ; Gois, P. M. P. ; Afonso, C. A. M. ; Trindade, A. F. Chem. Rev. 2009, 109, 2703.
(b) Beutner, G. L. ; Desai, L. ; Fanfair, D. ; Lobben, P. Anderson, E. ; Leung, S. W. ; Eastgate, M. D. Org. Process Res. Dev. 2014, 18, 1812.
(c) Xu, H. ; Zhou, B. ; Zhou, P. ; Zhou, J. ; Shen, Y. -H. ; Yu, F. -C. ; Lu, L. -L. Chem. Commun. 2016, 52, 8002.
(d) Grison, C. ; Genève, S. ; Coutrot, P. Tetrahedron Lett. 2001, 41, 3831.
(e) Kong, L. ; Yang, R. ; Du, X. ; Yan, S. ; Lin, J. Chin. J. Org. Chem. 2016, 36, 2437(in Chinese).
(孔令斌, 杨瑞霞, 杜璇璇, 严胜骄, 林军, 有机化学, 2016, 36, 2437. ) -
[15]
(a) Wang, M.-X.; Huang, Z.-T. J. Org. Chem. 1995, 60, 2807.
(b) Volodymyr, A. S.; Andriy, V. B.; Alexander, Y. P.; Mykhaylo, V. V. Synthesis 2007, 835. -
[16]
(a) Moya, A. D. ; Macía, C. A. ; García, T. M. I. ; Vélez, C. H. Synth. Commun. 1996, 26, 1187.
(b) Luo, D. ; Cui, S. ; Hu, X. ; Lin, J. ; Yan, S. Chin. J. Org. Chem. 2017, 37, 166(in Chinese). (罗大云, 崔时胜, 胡兴梅, 林军, 严胜骄, 有机化学, 2017, 37, 166. ) -
[17]
(a) Hubert, M.; Reinhard, T. Arch. Pharm. 1986, 319, 161.
(b) Hubert, M.; Reinhard, T. Arch. Pharm. 1987, 320, 1143. -
[18]
Jiang, X.-Y.; Liu, Z.-C.; Fang, L.; Yang, S.-J.; Lin, J. RSC Adv. 2014, 4, 26389. doi: 10.1039/C4RA02519A
-
[19]
(a) Li, M. ; Shao, P. ; Wang, S. -W. ; Kong, W. ; Wen, L. -R. J. Org. Chem. 2012, 77, 8956.
(b) Yu, F. -C. ; Huang, R. ; Ni, X. -C. ; Fan, J. ; Yan, S. -J. ; Lin, J. Green Chem. 2013, 15, 453.
(c) Yaqub, M. ; Arif, N. ; Perveen, R. ; Batool1, J. ; Riaz, M. T. ; Yaseen, M. Asian J. Org. Chem. 2015, 27, 1013.
(d) Chen, X. -B. ; Liu, Z. -C. ; Lin, X. -R. ; Huang, R. ; Yan, S. -J. ; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 2391.
(e) Chen, L. ; Huang, R. ; Du, X. -X. ; Yan, S. -J. ; Lin, J. ACS Sus-tainable Chem. Eng. 2017, 5, 1899.
(f) Yu, F. -C. ; Lin, X. -R. ; Liu, Z. -C. ; Zhang, J. -H. ; Liu, F. -F. ; Wu, W. ; Ma, Y. -L. ; Qu, W. -W. ; Yan, S. -J. ; Lin, J. ACS Omega 2017, 2, 873.
(g) Peng, M. ; Yang, R. ; Liu, X. ; Yan, S. ; Lin, J. Chin. J. Org. Chem. 2015, 35, 1754(in Chinese).
(彭美阳, 杨瑞霞, 刘昔敏, 严胜骄, 林军, 有机化学, 2015, 35, 1754. ) -
[20]
(a) Yu, F. -C. ; Yan, S. -J. ; Hu, L. ; Wang, Y. -C. ; Lin, J. Org. Lett. 2011, 13, 4782.
(b) Yu, F. -C. ; Hao, X. -P. ; Lin, X. -R. ; Yan, S. -J. ; Lin, J. Tetrahedron 2015, 71, 4084.
(c) Li, M. ; Zhou, Z. -M. ; Wen, L. -R. ; Qiu, Z. -X. J. Org. Chem. 2011, 76, 3054.
(d) Du, X. -X. ; Huang, R. ; Yang, C. -L. ; Lin, J. ; Yan, S. -J. RSC Adv. 2017, 7, 40067.
(e) Ma, Y. -L. ; Wang, K. -M. ; Huang, R. ; Lin, J. ; Yan, S. -J. Green Chem. 2017, 19, 3574.
(f) Cui, S. -S. ; Huang, R. ; Luo, D. -Y. ; Yan, S. -J. ; Lin, J. Eur. J. Org. Chem. 2017, 3442.
(g) Yang, R. ; Zhao, Y. ; Jiang, M. ; Yan, S. ; Lin, J. Chin. J. Org. Chem. 2016, 36, 2941(in Chinese).
(杨瑞霞, 赵宇澄, 蒋美妤, 严胜骄, 林军, 有机化学, 2016, 36, 2941. )
(h) Chen, L. ; Wang, B. ; Zhao, Y. ; Yan, S. ; Lin, J. Chin. J. Org. Chem. 2017, 37, 1433(in Chinese).
(陈亮, 王保取, 赵宇澄, 严胜骄, 林军, 有机化学, 2017, 37, 1433. ) -
[21]
(a) Meziane, M. A.; Rahmouni, M.; Bazureau, J. P.; Hamelin, J. Synthesis 1998, 967.
(b) Dahmani, Z. M.; Rahmouni, R.; Brugidou, J. P.; Bazureau, J. Tetrahedron Lett. 1998, 39, 8453.
(c) Schafer, H.; Gruner, M.; Grobmann, G.; Gewald, K. Monatsh. Chem. 1991, 122, 959. -
[22]
Papmeyer, M.; Vuilleumier, C. A.; Pavan, G. M.; Zhurov, K. O.; Severin, K. Angew. Chem., Int. Ed. 2016, 55, 1685. doi: 10.1002/anie.201510423
-
[23]
Schirok, H.; Alonso-Alija, C.; Benet-Buchholz, J.; Goeller, A. H.; Grosser, R.; Michels, M.; Paulsen, H. J. Org. Chem. 2005, 70, 9463. doi: 10.1021/jo0515428
-
[24]
(a) Kelly-Rowley, A. M.; Lynch, V. M.; Anslyn, E. V. J. Am. Chem. Soc. 1995, 117, 3438.
(b) Kelly-Rowley, A. M.; Cabell, L. A.; Anslyn, E. V. J. Am. Chem. Soc. 1991, 113, 9687.
(c) Alizadeh, A.; Zarei, A.; Rezvanian, A. Synthesis 2011, 497. -
[25]
(a) Maryamabadi, A.; Hasaninejad, A.; Nowrouzi, N.; Mohbbi, G.; Asghari, B. Bioorg. Med. Chem. 2016, 24, 1408.
(b) Maryamabadi, A.; Hasaninejad, A.; Nowrouzi, N.; Mohebbi, G. Bioorg. Med. Chem. 2017, 25, 2507. -
[26]
Silva, R. C.; Silva, P. G.; Sangi, D. P.; Pontes, J. G.; Ferreira, A. G.; Corrêa, A. G.; Paixão, M. W. Tetrahedron 2013, 69, 9007. doi: 10.1016/j.tet.2013.08.040
-
[27]
Liu, J.; Zhang, H.-R.; Lin, X.-R.; Yan, S.-J.; Lin, J. RSC. Adv. 2014, 4, 27582. doi: 10.1039/C4RA03863K
-
[28]
Safron, S. A.; King, G. A.; Horvat, R. C. J. Am. Chem. Soc. 1981, 103, 6333. doi: 10.1021/ja00411a012
-
[29]
(a) Cassani, C.; Bergonzini, G.; Wallentin, C.-J. Org. Lett. 2014, 16, 4228.
(b) Tanner, D. D.; Osman, S. A. A. J. Org. Chem. 1987, 21, 4689.
-
[1]
-
表 1 反应条件的优化a
Table 1. Optimization of reaction conditions

Entry Catalyst Solvent T/℃ Time/h Yieldb /% 1 — 1, 4-Dioxane r.t. 8 — 2 — 1, 4-Dioxane Reflux 8 — 3 Et3Nc 1, 4-Dioxane Reflux 2 56 4 Piperidinec 1, 4-Dioxane Reflux 2 12 5 Cs2CO3 d 1, 4-Dioxane Reflux 2 5 6 t-BuOKd 1, 4-Dioxane Reflux 2 — 7 AcOHc 1, 4-Dioxane Reflux 2 — 8 Et3Nc EtOH Reflux 2 — 9 Et3Nc THF Reflux 2 — 10 Et3Nc MeCN Reflux 2 — 11 Et3Nc DMF 100 2 23 12 Et3Nc Toluene Reflux 2 41 13 Et3N c 1, 4-Dioxane Reflux 4 68 14 Et3Nc 1, 4-Dioxane Reflux 8 67 aThe reaction was performed with 1a (1.0 mmol), 2 (2.0 mmol).bIsolated yields based on 1a.c The catalyst 0.05 mmol.d The catalyst 2.0 mmol. 表 2 亚胺基吡咯酮类化合物3a~3l的合成
Table 2. Synthesis of iminopyrrolone derivatives 3a~3l

表 3 化合物3c的晶体数据及结构修正
Table 3. Crystal data and structure refinement for compound 3c
Empirical formula C19H16Cl2N2O Formula weight 359.24 Temperature 293.15 K Wavelength 0.71073 Å Crystal system, space group Triclinic, P-1 Unit cell dimensions a=7.5752(12) Å b=10.7338(17) Å c=12.270(3) Å α=112.537(3)° β=105.009(3)° γ=95.266(2)° Volume 869.4(3) Å3 Z, Calculated density 2, 1.372 Mg/m3 F(000) 372.0 Crystal size 0.1 mm×0.28 mm×0.42 mm Theta range for data collection 3.794° to 50.294° Limiting indices -9≤h≤9 -12≤k≤12, -14≤l≤14 Reflection collected 6847 independent reflections 3084 [Rint=0.0199, Rσ=0.0261] Data/restraints/parameters 3084/0/227 Absorption correction Semi-empirical from equivalents Refinement method Full-matrix least-squares on F2 Goodness-of-fit on F2 1.069 Final R indices [I>2σ(I)] R1=0.0349, wR2=0.0898 Final R indexes [all data] R1=0.0407, wR2=0.0951 Largest diff. peak and hole 0.18/-0.32 A-3 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 9
- 文章访问数: 2223
- HTML全文浏览量: 309





 下载:
下载:



 下载:
下载:

