 图 图式1
目标化合物的合成
Figure 图式1.
Synthetic route of the target compounds
图 图式1
目标化合物的合成
Figure 图式1.
Synthetic route of the target compounds

Citation: Pan Xiaole, Wang Lei, Dun Yanyan, Fang Hao. Synthesis and Antiproliferative Activity Studies on 2-Substitued Aniline Quinazoline Derivatives[J]. Chinese Journal of Organic Chemistry, 2016, 36(5): 1044-1050. doi: 10.6023/cjoc201510018

2-取代苯氨基喹唑啉衍生物的合成及抗肿瘤活性研究
English
Synthesis and Antiproliferative Activity Studies on 2-Substitued Aniline Quinazoline Derivatives
-
Key words:
- quinazoline derivatives
- / antitumor activity
- / synthesis
-
喹唑啉类化合物最早从Gabriel植物中分离提取得到,随后发现其具有镇静[1, 2]、抗炎[3]、抗高血压[4~6]、抗疟[7]和抗真菌[8~10]等多种药理作用. 近年来,喹唑啉骨架作为药效活性片段,已在抗肿瘤药物的研发中不断被成功应用[11~13]. 到目前为止,已有Albaconazole[14, 15]、Gefitinib[16]、Erlotinib[17]、Vandetanib[18]等多个喹唑啉衍生物结构作为抗肿瘤药物上市,被应用于肺癌和胰腺癌等恶性肿瘤的临床治疗[19~21].
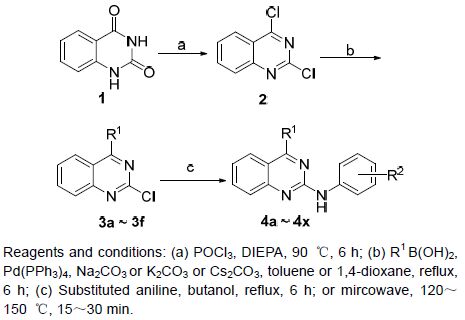
本课题组近期曾针对国外Ⅱ期临床研究的药物R-Roscovitine进行结构改造[22]. 前期针对该药的构效关系研究主要基于其嘌呤环(嘧啶并咪唑)母核,在嘌呤环2位和4位引入不同的芳胺或脂肪胺片断[23]. 根据药物设计学原理,我们在保留R-Roscovitine母核嘧啶环的基础上,将咪唑环以苯环替代,设计合成了一系列2-取代芳胺喹唑啉类衍生物,并进行构效关系研究. 合成路线设计见Scheme 1.
Compd. R1 R2 4a Ph H 4b Ph 4-CH3 4c Ph 3-NO2 4d Ph 3-Morpholino 4e Ph 4-Morpholino 4f Ph 4-SO2NH2 4g 4-CH3C6H4 4-CH3 4h 4-CH3C6H4 3-NO2 4i 4-CH3C6H4 4-Morpholino 4j 3-NO2C6H4 H 4k 3-NO2C6H4 4-CH3 4l 3-NO2C6H4 3-NO2 4m 3-NO2C6H4 4-Morpholino 4n Thiophen-3-yl H 4o Thiophen-3-yl 4-CH3 4p Thiophen-3-yl 3-NO2 4q Thiophen-3-yl 4-Morpholino 4r Thiophen-3-yl 4-CH3 4s Thiophen-3-yl 3-NO2 4t Thiophen-3-yl 4-Morpholino 4u Furan-2-yl H 4v Furan-2-yl 4-CH3 4w Furan-2-yl 3-NO2 4x Furan-2-yl 4-Morpholino 表 1 化合物4的结构
Table 1. The structure of the compounds 41 结果与讨论
1.1 目标化合物的合成
化合物4a~4x的合成是以2,4-喹唑啉二酮为起始原料,首先经过三氯氧磷的处理得到2,4-二氯喹唑啉(2),然后与各种芳香硼酸发生Suzuki偶联得关键中间体3a~3f,最后在N2的保护下或者微波条件下与各种取代的苯胺反应得到终产物4a~4x. 目标化合物的结构均经1H NMR,13C NMR和HRMS确证.
1.2 目标化合物的抗肿瘤活性
所有目标化合物浓度在50 μmol/L条件下,应用Hela肿瘤细胞株对其进行体外抗增殖活性测试,结果见图 1. 实验结果说明,喹唑啉C(4)位引入3-硝基苯基(如化合物4j、4k、4l和4m)、3-噻吩基(如化合物4o、4p和4q)及2-噻吩基(如化合物4r、4s和4t)的活性较差; 喹唑啉C(2)位的苯胺若带有硝基或者吗啉基(如化合物4h、4m、4p、4w和4x)不利于提高其对肿瘤细胞的抑制作用,而在喹唑啉的C(4)位引入2-呋喃基、C(2)位引入苯胺和对甲基苯胺时(如化合物4u和4v),目标化合物的抗肿瘤活性得到明显改善.
我们选取活性化合物4u和4v(抑制率大于80%)并测试其对Hela、A549和MCF-7三种肿瘤细胞株的抗增殖效果(Table 2). 结果显示,两个活性化合物抑制Hela、A549肿瘤细胞株的活性与阳性对照药R-Roscovitine相当或优于阳性对照药R-Roscovitine,对MCF-7肿瘤细胞株的抑制作用不如阳性对照药R-Roscovitine. 分析两个活性化合物对不同瘤株的抗增殖效果,可发现它们对于Hela肿瘤细胞的抑制作用明显好于其它两株肿瘤细胞A549和MCF-7.
Compd. IC50/(μmol•L-1) Hela A549 MCF-7 4u 12.76±2.36 32.95±1.17 16.21±2.00 4v 14.93±1.59 30.47±1.56 26.24±0.76 R-Roscovitine 14.01±2.62 78.70±6.03 12.72±1.25 表 2 代表化合物的体外抗增殖活性
Table 2. Antiproliferative activities of representative compounds2 结论
本文以R-Roscovitine为先导化合物并结合喹唑啉骨架在肿瘤药物研发的应用,根据药物设计学原理,设计并合成了24个结构全新的目标化合物. 所有目标化合物的结构均经1H NMR,13C NMR和HRMS确证. 在体外抗肿瘤细胞增殖活性的试验中,有多个化合物在浓度为50 μmol/L时对Hela肿瘤细胞株都有明显的抑制作用; 其中初筛时活性最好的化合物4u、4v对Hela、A549两株肿瘤细胞抗增殖作用与阳性对照药R-Roscovitine相当或优于R-Roscovitine,对MCF-7肿瘤细胞株的抗增殖作用不如阳性对照药R-Roscovitine. 上述结果为今后研究新型喹唑啉类抗肿瘤药物奠定了良好基础.
3 实验部分
3.1 仪器与试剂
合成中所用到的原料均为市售的国产试剂,纯度为96%~99%; 反应溶剂为分析纯,未经处理直接使用. 人子宫颈癌细胞Hela、人乳腺癌细胞MCF-7、人肺腺癌细胞A549; 改良型RPMI1640培养基(美国Hyclone公司),2.5 g/L胰蛋白酶(美国Gibco公司),10%(体积分数)胚牛血清(美国Hyclone公司). 阳性对照药R-Roscovatine购自MedChemExpress (MCE)中国分公司.
Bruker-300 MHz和Bruker-400 MHz核磁共振波谱仪; Agilent 6520型高分辨电喷雾电离质谱仪; RY-1型熔点仪(天津市分析仪器厂); Thermo Varioskan Flash全波长多功能酶标仪; Thermo Forma 3111型二氧化碳恒温培养箱; Olympus CKX31双目倒置显微镜.
3.2 化合物的合成
3.3 抗肿瘤活性实验
采用四甲基偶氮唑盐(MTT)法对24个目标化合物进行体外抗肿瘤活性评价. 测试细胞为Hela、A549和MCF-7.
将处于对数生长期的肿瘤细胞,用含10%(体积分数)胚牛血清的RPMI1640培养液调整细胞数(所选细胞先用2.5×10-3 g/mL胰蛋白酶消化后调整为细胞数4×104 cell/mL的细胞悬液),接种于96孔培养板中,37 ℃、5% CO2条件下培养8 h后,加入不同浓度的待测化合物,继续培养48 h. 然后每孔加入10 μL MTT (5 mg/mL),继续培养4 h后,弃去孔中液体,每孔加入150 μL DMSO,37 ℃恒温振摇5~10 min,检测570 nm波长处的OD值,计算所测化合物对细胞的抑制率以及IC50值.
辅助材料(Supporting Information) 所有化合物的1H NMR、13C NMR和HRMS谱图. 这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
3.2.2 目标化合物4a~4x的合成
将化合物3 (1 mmol)与各种取代的苯胺(1 mmol)混合,加入正丁醇或二甲亚砜(4~6 mL),在氮气保护下110~120 ℃反应6~8 h(或反应混合物微波加热110~150 ℃反应15~45 min),TLC监测至反应结束. 反应液冷至室温,静置12 h后过滤,滤渣经柱层析得到目标化合物4a~4x.
N,4-二苯基喹唑啉-2-胺(4a): 黄色固体,产率75%. m.p. 133~134 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 9.93 (s,1H,NH),8.04 (d,J=7.8 Hz,2H,ArH),7.85~7.74 (m,5H,ArH),7.65~7.61 (m,3H,ArH),7.38~7.31 (m,3H,ArH),6.98 (t,J=7.2 Hz,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 169.80,156.78,152.63,141.19,137.11,134.60,130.31,129.96,128.98,128.90,127.45,126.74,124.05,121.93,119.22,118.78; HRMS (AP-ESI) calcd for C20H16N3 [M+H]+ 298.1344,found 298.1335.
4-苯基-N-(4-甲基苯基)喹唑啉-2-胺(4b): 黄色固体,产率55%. m.p. 137~139 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.86 (s,1H,NH),7.90 (d,J=8.4 Hz,2H,ArH),7.83~7.72 (m,5H,ArH),7.63~7.61 (m,3H,ArH),7.35~7.31 (m,1H,ArH),7.15 (d,J=8.0 Hz,2H,ArH),2.28 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ:169.81,156.79,152.56,138.55,137.14,134.57,130.84,130.30,129.94,129.40,128.89,127.46,126.55,123.87,119.44,118.68,20.92; HRMS (AP-ESI) calcd for C21H18N3 [M+H]+ 312.1501,found 312.1495.
N-(3-硝基苯基)-4-苯基喹唑啉-2-胺(4c): 黄色固体,产率89%. m.p. 166~168 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 10.54 (s,1H,NH),9.21 (s,1H,ArH),8.32 (d,J=8.0 Hz,1H,ArH),7.92~7.88 (m,2H,ArH),7.85~7.80 (m,4H,ArH),7.65~7.60 (m,4H,ArH),7.46 (t,J=8.0 Hz,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 170.54,155.93,151.31,148.61,142.09,136.75,135.20,130.69,130.25,130.14,128.99,127.71,126.19,125.38,125.03,118.99,116.49,113.32; HRMS (AP-ESI) calcd for C20H15N4O2 [M+H]+ 343.1195,found 343.1192.
N-(3-吗啉基苯基)-4-苯基喹唑啉-2-胺(4d): 红色固体,产率73%. m.p. 154~156 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 10.16 (s,1H,NH),8.04 (s,1H,ArH),7.89~7.78 (m,5H,ArH),7.65~7.61 (m,3H,ArH),7.45~7.38 (m,2H,ArH),7.30 (t,J=8.0 Hz,1H,ArH),6.85 (s,1H,ArH),3.87 (br s,4H,O(CH2)2),3.26 (br s,4H,N(CH2)2); 13C NMR (100 MHz,DMSO-d6) δ: 171.74,154.95,148.92,140.69,136.53,135.43,130.86,130.07,129.94,128.86,127.96,124.86,118.30,112.30,109.53,65.54,51.47; HRMS (AP-ESI) calcd for C24H23N4O [M+H]+ 383.1872,found 383.1866.
N-(4-吗啉基苯基)-4-苯基喹唑啉-2-胺(4e): 黄色固体,产率70%. m.p. 152~154 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.72 (s,1H,NH),7.87 (d,J=8.8 Hz,2H,ArH),7.80~7.74 (m,4H,ArH),7.69 (d,J=8.4 Hz,1H,ArH),7.62~7.61 (m,3H,ArH),7.31 (t,J=8.0 Hz,1H,ArH),6.96 (d,J=8.8 Hz,2H,ArH),3.76 (d,J=4.4 Hz,4H,O(CH2)2),3.07 (d,J=4.4 Hz,4H,N(CH2)2). 13C NMR (100 MHz,DMSO-d6) δ: 169.09,156.41,152.38,146.15,136.71,133.97,133.13,129.72,129.40,128.37,126.93,126.04,123.02,119.96,118.09,115.62,66.17,49.27; HRMS (AP-ESI) calcd for C24H23N4O [M+H]+ 383.1872,found 383.1861.
4-[(4-苯基喹唑啉-2-基)胺基]苯磺酰胺(4f): 黄色固体,产率64%. m.p. 246~248 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 10.38 (s,1H,NH),8.20 (d,J=8.8 Hz,2H,ArH),7.90~7.77 (m,7H,ArH),7.65~7.63 (m,3H,ArH),7.44~7.40 (m,1H,ArH),7.22 (s,2H,SO2NH2); 13C NMR (100 MHz,DMSO-d6) δ: 169.59,155.85,151.79,143.75,136.43,136.27,134.34,129.98,129.55,128.47,127.04,126.56,126.47,124.29,118.60,117.92; HRMS (AP-ESI) calcd for C20H17N4O2S [M+H]+ 377.1072,found 377.1061.
N,4-二(4-甲基苯基)喹唑啉-2-胺(4g): 黄色固体,产率76%. m.p. 218~220 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 10.01 (s,1H,NH),7.86~7.80 (m,4H,ArH),7.75~7.66 (m,3H,ArH),7.45 (d,J=8.1 Hz,2H,ArH),7.38 (t,J=7.8 Hz,1H,ArH),7.17 (d,J=8.4 Hz,2H,ArH),2.45 (s,3H,CH3),2.29 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 172.80,154.39,147.05,141.38,136.15,133.61,133.47,130.37,129.81,129.69,128.50,125.15,122.60,121.58,118.18,21.53,21.00; HRMS (AP-ESI) calcd for C22H20N3 [M+H]+ 326.1657,found 326.1653.
N-(3-硝基苯基)-4-(4-甲基苯基)喹唑啉-2-胺(4h): 黄色固体,产率51%. m.p. 201~203 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 10.48 (s,1H,NH),9.21 (s,1H,ArH),8.32 (dd,J=8.1,1.2 Hz,1H,ArH),7.93~7.86 (m,2H,ArH),7.84~7.79 (m,2H,ArH),7.74 (d,J=8.1 Hz,2H,ArH),7.64 (t,J=8.1 Hz,1H,ArH),7.46~7.40 (m,3H,ArH),2.46 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 170.44,156.01,151.44,148.64,142.18,140.57,135.14,134.00,130.29,130.22,129.59,127.80,126.25,125.38,124.99,119.04,116.46,113.30,21.50; HRMS (AP-ESI) calcd for C21H17N4O2 [M+H] + 357.1352,found 357.1354.
N-(4-吗啉基苯基)-4-(4-甲基苯基)喹唑啉-2-胺(4i): 黄色固体,产率18%. m.p. 159~161 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.69 (s,1H,NH),7.87 (d,J=8.8 Hz,2H,ArH),7.80~7.77 (m,2H,ArH),7.67~7.65 (m,3H,ArH),7.43 (d,J=8 Hz,2H,ArH),7.30 (t,J=8 Hz,1H,ArH),6.95 (d,J=9.2 Hz,2H,ArH),3.76 (t,J=4.4 Hz,4H,O(CH2)2),3.07 (t,J=4.4 Hz,4H,N(CH2)2),2.44 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 169.51,156.95,152.91,146.63,139.95,134.43,134.38,133.68,129.95,129.45,127.48,126.52,123.44,120.47,118.63,116.12,66.67,49.79,21.46; HRMS (AP-ESI) calcd for C25H25N4O [M+H]+ 397.2028,found 397.2025.
4-(3-硝基苯基)-N-苯基喹唑啉-2-胺(4j): 黄色固体,产率71%. m.p. 178~179 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 10.03 (s,1H,NH),8.60 (t,J=2.1 Hz,1H,ArH),8.50~8.46 (m,1H,ArH),8.27 (dt,J=7.5,0.9 Hz,1H,ArH),8.04 (d,J=7.8 Hz,2H,ArH),7.95~7.78 (m,4H,ArH),7.41~7.32 (m,3H,ArH),7.02 (t,J=7.5 Hz,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 167.43,156.64,152.77,148.32,141.03,138.50,136.48,134.94,130.64,128.99,126.99,126.88,125.03,124.72,124.41,122.08,119.27,118.51; HRMS (AP-ESI) calcd for C20H15N4O2 [M+H]+ 343.1195,found 343 .1194.
4-(3-硝基苯基)-N-(4-甲基苯基)喹唑啉-2-胺(4k): 红色固体,产率56%. m.p. 184~186 ℃; 1H NMR (400 MHz,CDCl3) δ: 11.25 (brs,1H,NH),8.67 (s,1H,ArH),8.55 (d,J=7.6 Hz,1H,ArH),8.15 (d,J=6.8 Hz,1H,ArH),7.99~7.94 (m,3H,ArH),7.86 (t,J=7.6 Hz,1H,ArH),7.55 (d,J=7.6 Hz,3H,ArH),7.22 (d,J=7.6 Hz,2H,ArH),2.36 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 169.16,155.38,149.53,148.31,138.07,136.99,136.49,135.86,132.55,130.79,129.66,127.55,125.43,124.95,124.81,124.49,120.68,118.24,20.96; HRMS (AP-ESI) calcd for C21H17N4O2 [M+H]+357.1352,found 357.1344.
N,4-二(-3-硝基苯基)喹唑啉-2-胺(4l): 黄色固体,产率26%. m.p. 236~238 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 10.59 (s,1H,NH),9.20 (t,J=2.1 Hz,1H,ArH),8.64 (t,J=2.1 Hz,1H,ArH),8.52~8.48 (m,1H,ArH),8.34~8.27 (m,2H,ArH),7.97~7.84 (m,5H,ArH),7.66 (t,J=8.4 Hz,1H,ArH),7.50~7.44 (m,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 167.80,156.23,152.36,148.66,148.38,142.33,138.32,136.57,135.32,130.72,130.29,127.16,127.02,125.21,125.08,124.84,118.91,116.32,112.96; HRMS (AP-ESI) calcd for C20H14N5O4 [M+H] + 388.1046,found 388.1040.
N-(4-吗啉基苯基)-4-(3-硝基苯基)喹唑啉-2-胺(4m): 红色固体,产率19%. m.p. 144~146 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.83 (s,1H,NH),8.58 (t,J=2.0 Hz,1H,ArH),8.49 (dd,J=8.0,1.2 Hz,1H,ArH),8.25 (d,J=8 Hz,1H,ArH),7.93 (t,J=8 Hz,1H,ArH),7.87~7.84 (m,2H,ArH),7.82~7.80 (m,1H,ArH),7.79 (d,J=8.4 Hz,1H,ArH),7.72 (d,J=8 Hz,1H,ArH),7.35 (t,J=7.2 Hz,1H,ArH),6.97 (d,J=8.8 Hz,2H,ArH),3.77 (t,J=4.8 Hz,4H,O(CH2)2),3.08 (t,J=4.8 Hz,4H,N(CH2)2); 13C NMR (100 MHz,DMSO-d6) δ: 167.24,156.78,153.04,148.32,146.77,138.62,136.44,134.82,133.44,130.61,126.97,126.68,124.96,124.67,123.88,120.52,118.32,116.09,66.66,49.73; HRMS (AP-ESI) calcd for C24H22N5O3 [M+H]+ 428.1723,found 428.1714.
N-苯基-4-(噻吩-3-基)喹唑啉-2-胺(4n): 黄色固体,产率50%. m.p. 126~128 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.84 (s,1H,NH),8.22 (dd,J=3.2,1.2 Hz,1H,ArH),8.09 (d,J=8.0 Hz,1H,ArH),8.02 (d,J=8.0 Hz,2H,ArH),7.84~7.80 (m,2H,ArH),7.74 (d,J=8.0 Hz,1H,ArH),7.66 (dd,J=5.2,1.2 Hz,1H,ArH),7.40~7.31 (m,3H,ArH),6.70 (t,J=7.2 Hz,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 164.51,156.85,152.76,141.22,138.42,134.55,129.60,129.41,128.97,127.37,127 .25,126.71,124.14,121.89,119.26,118.83; HRMS (AP-ESI) calcd for C18H14N3S [M+H]+ 304.0908,found 304.0901.
4-(噻吩-3-基)-N-(4-甲基苯基)喹唑啉-2-胺(4o): 砖红色固体,产率54%. m.p. 224~226 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 9.91 (s,1H,NH),8.25~8.24 (m,1H,ArH),8.10 (d,J=8.1 Hz,1H,ArH),7.86~7.80 (m,4H,ArH),7.73 (d,J=8.1 Hz,1H,ArH),7.65 (dd,J=5.1,1.2 Hz,1H,ArH),7.42 (t,J=7.8 Hz,1H,ArH),7.17 (d,J=8.4 Hz,2H,ArH),2.29 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 167.17,154.19,137.75,136.35,135.88,133.68,132.39,129.85,129.41,128.28,128.05,125.46,121.74,118.01,21.02; HRMS (AP-ESI) calcd for C19H16N3S [M+H]+ 318.1065,found 318.1050.
N-(3-硝基苯基)-4-(噻吩-3-基)喹唑啉-2-胺(4p): 黄色固体,产率89%. m.p. 205~207 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 10.99 (s,1H,NH),9.77 (t,J=2.1 Hz,1H,ArH),8.87~8.84 (m,2H,ArH),8.74 (d,J=8.4 Hz,1H,ArH),8.49~8.34 (m,4H,ArH),8.27 (dd,J=5.4,1.2 Hz,1H,ArH),8.21 (t,J=8.1 Hz,1H,ArH),8.06~8.01 (m,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 165.16,155.84,151.16,148.59,142.01,138.09,135.23,130.65,130.24,129.37,127.65,127.54,125.92,125.48,125.18,118.92,116.53,113.43; HRMS (AP-ESI) calcd for C18H13N4O2S [M+H]+ 349.0759,found 349.0751.
N-(4-吗啉基苯基)-4-(噻吩-3-基)喹唑啉-2-胺(4q): 黄色固体,产率8%. m.p. 210~212 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.69 (s,1H,NH),8.25 (dd,J=2.4,0.8 Hz,1H,ArH),8.09 (d,J=8 Hz,1H,ArH),7.91 (d,J=8.8 Hz,2H,ArH),7.86~7.81 (m,2H,ArH),7.72~7.67 (m,2H,ArH),7.40 (t,J=0.8 Hz,1H,ArH),7.01 (d,J=9.2 Hz,2H,ArH),3.81 (t,J=4.4 Hz,4H,O(CH2)2), 3.12 (t,J=4.4 Hz,4H,N(CH2)2); 13C NMR (100 MHz,DMSO-d6) δ: 164.35,156.98,152.99,146.64,138.51,134.45,133.66,129.43,129.40,127.32,127.25,126.51,123.63,120.50,118.65,116.13,66.67,49.78; HRMS (AP-ESI) calcd for C22H21N4OS [M+H]+ 389.1436,found 389.1430.
4-(噻吩-2-基)-N-(4-甲基苯基)喹唑啉-2-胺(4r): 红色固体,产率59%. m.p. 206~208 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 10.13 (s,1H,NH),8.41 (d,J=8 Hz,1H,ArH),8.09 (d,J=3.6 Hz,1H,ArH),8.05 (d,J=5.2 Hz,1H,ArH),7.90~7.87 (m,1H,ArH),7.77~7.73 (m,3H,ArH),7.49 (t,J=14.8 Hz,1H,ArH),7.39 (dd,J=4.8,3.6 Hz,1H,ArH),7.21 (d,J=8.4 Hz,2H,ArH),2.31 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 164.64,153.06,145.47,140.21,136.78,135.47,134.97,133.98,129.93,129.82,127.98,125.95,121 .85,121.35,116.70,21.02; HRMS (AP-ESI) calcd for C19H16N3S [M+H]+ 318.1065,found 318.1058.
N-(3-硝基苯基)-4-(噻吩-2-基)喹唑啉-2-胺(4s): 黄色固体,产率16%. m.p. 169~171 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 10.37 (s,1H,NH),9.18 (t,J=2.4 Hz,1H,ArH),8.43 (d,J=8 Hz,1H,ArH),8.30 (dd,J=8.4,1.6 Hz,1H,ArH),8.07 (dd,J=3.6,0.8 Hz,1H,ArH),8.03 (dd,J=5.2,1.2 Hz,1H,ArH),7.93 (m,1H,ArH),7.85 (dd,J=8,1.6 Hz,1H,ArH),7.79 (d,J=8 Hz,1H,ArH),7.64 (t,J=8 Hz,1H,ArH),7.53~7.49 (m,1H,ArH),7.39~7.37 (m,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 161.74,156.05,152.89,148.62,142.46,140.56,134.93,132.54,131.96,130.12,129.16,126.99,126.94,125.19,125.11,117.93,116.08,113.03; HRMS (AP-ESI) calcd for C18H13N4O2S [M+H]+ 349.0759,found 349.0757.
N-(4-吗啉基苯基)-4-(噻吩-2-基)喹唑啉-2-胺(4t): 橙色固体,产率36%. m.p. 229~231 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.57 (s,1H,NH),8.32 (d,J=8.4 Hz,1H,ArH),7.99 (d,J=3.6 Hz,1H,ArH),7.96 (d,J=5.2 Hz,1H,ArH),7.84~7.78 (m,3H,ArH),7.66 (d,J=8 Hz,1H,ArH),7.39~7.33 (m,2H,ArH),6.96 (d,J=8.8 Hz,2H,ArH),3.76 (t,J=4.4 Hz,4H,O(CH2)2),3.07 (t,J=4.4 Hz,4H,N(CH2)2); 13C NMR (100 MHz,DMSO-d6) δ: 161.37,156.68,153.53,146.69,140.88,134.59,133.54,132.01,131.42,129.01,126.83,126.69,123.96,120.64,117.47,116.08,66.67,49.76; HRMS (AP-ESI) calcd for C22H21N4OS [M+H]+ 389.1436,found 389.1431.
4-(呋喃-2-基)-N-苯基喹唑啉-2-胺(4u): 黄色固体,产率14%. m.p. 126~128 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.84 (s,1H,NH),8.62 (d,J=8 Hz,1H,ArH),8.17 (d,J=1.2 Hz,1H,ArH),8.02 (d,J=8 Hz,2H,ArH),7.85~7.81 (m,1H,ArH),7.72 (d,J=8.4 Hz,1H,ArH),7.57 (d,J=3.2 Hz,1H,ArH),7.45~7.41 (m,1H,ArH),7.36 (t,J=7.6 Hz,2H,ArH),7.01 (t,J=7.6 Hz,1H,ArH),6.88~6.86 (m,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 156.7 9,156.43,153.42,152.23,147.25,141.23,134.61,128.98,126.92,126.89,124.41,121.86,119.22,116.89,116.60,113.07; HRMS (AP-ESI) calcd for C18H14N3O [M+H]+ 288.1137,found 288.1134.
4-(呋喃-2-基)-N-(4-甲基苯基)喹唑啉-2-胺(4v): 黄色固体,产率60%. m.p. 124~126 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.83 (s,1H,NH),8.61 (d,J=8 Hz,1H,ArH),8.17 (s,1H,ArH),7.85~7.80 (m,3H,ArH),7.69 (d,J=8.4 Hz,1H,ArH),7.56 (d,J=3.2 Hz,1H,ArH),7.42 (t,J=7.5 Hz,1H,ArH),7.16 (d,J=7.6 Hz,2H,ArH),6.87 (dd,J=3.2,1.5 Hz,1H,ArH),2.29 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6) δ: 157.70,153.43,151.24,148.75,135.73,135.39,132.77,129.20,127.14,124.96,121.76,120.82,119.37,115.47,113.25,20.43; HRMS (AP-ESI) calcd for C19H16N3O [M+H]+ 302.1293,found 302.1285.
4-(呋喃-2-基)-N-(3-硝基苯基)喹唑啉-2-胺(4w): 黄色固体,产率22%. m.p. 180 (dec.) ℃; 1H NMR (400 MHz,DMSO-d6) δ: 10.40 (s,1H,NH),9.23 (t,J=2 Hz,1H,ArH),8.70 (d,J=8 Hz,1H,ArH),8.26 (d,J=7.6 Hz,1H,ArH),8.20 (d,J=0.8 Hz,1H,ArH),7.91~7.87 (m,1H,ArH),7,84 (dd,J=8,1.6 Hz,1H,ArH),7.77 (d,J=8.4 Hz,1H,ArH),7.66~7.60 (m,2H,ArH),7.53 (t,J=8 Hz,1H,ArH),6.91~6.89 (m,1H,ArH); 13C NMR (100 MHz,DMSO-d6) δ: 156.42,156.34,153.10,152.24,148.61,147.53,142.55,134.82,130.11,126.97,125.08,124.96,117.10,116.96,115.95,113.16,112.84; HRMS (AP-ESI) calcd for C18H13N4O3 [M+H]+ 333.0988,found 333.0981.
4-(呋喃-2-基)-N-(4-吗啉基苯基)喹唑啉-2-胺(4x): 黄色固体,产率69%. m.p. 235 (dec.) ℃; 1H NMR (400 MHz,DMSO-d6) δ: 9.61 (s,1H,NH),8.57 (d,J=8 Hz,1H,ArH),8.15 (d,J=1.2 Hz,1H,ArH),7.85 (d,J=8.8 Hz,2H,ArH),7.80 (td,J=8.4,1.2 Hz,1H,ArH),7.64 (d,J=8 Hz,1H,ArH),7.52 (d,J=3.2 Hz,1H,ArH),7.39~7.35 (m,1H,ArH),6.96 (d,J=8.8 Hz,2H,ArH),6.86~6.85 (m,1H,ArH),3.76 (t,J=4.4 Hz,4H,O(CH2)2),3.07 (t,J =4.4 Hz,4H,N(CH2)2); 13C NMR (100 MHz,DMSO- d6) δ: 156.92,156.33,153.63,152.30,147.09,146.64,134.49,133.66,126.87,126.71,123.89,120.49,116.74,116.35,116.12,112.99,66.67,49.78; HRMS (AP-ESI) calcd for C22H21N4O2 [M+H]+ 373.1665,found 373.1657.
3.2.1 中间体2及3a~3f的合成
中间体3a~3f的合成. 将化合物2 (0.20 g,1 mmol)、R1B(OH)2 (1 mmol)、Na2CO3或K2CO3或Cs2CO3 (3 mmol)、Pd(PPh3)4 (0.03~0.05 mmol)置于50 mL两颈瓶中,以甲苯(或二氧六环等)为溶剂,氮气(N2)保护下于90~110 ℃反应. 用薄层色谱(TLC)监测反应,反应结束后向反应体系中加适量水并用EtOAc萃取、硫酸镁干燥、浓缩或者直接过滤然后用减压蒸馏蒸干溶剂,经柱层析得到所要产物.
2-氯-4-苯基喹唑啉(3a): 白色固体,产率78%. m.p. 108~110 ℃(文献值[26] 112~114 ℃); 1H NMR (300 MHz,DMSO-d6) δ: 8.14~8.04 (m,3H,ArH),7.84~7.77 (m,3H,ArH),7.71~7.62 (m,3H,ArH).
2-氯-4-(4-甲基苯基)喹唑啉(3b): 白色固体,产率72%. m.p. 132~134 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 8.16 (d,J=8.7 Hz,1H,ArH),8.11~8.03 (m,2H,ArH),7.80~7.72 (m,3H,ArH),7.48 (d,J=8.1 Hz,2H,ArH),2.46 (s,3H,CH3). HRMS (AP-ESI) calcd for C15H12ClN2 [M+H]+ 255.0689,found 255.0690.
2-氯-4-(3-硝基苯基)喹唑啉(3c): 白色固体,产率53%. m.p. 190~192 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 8.61 (t,J=1.8 Hz,1H,ArH),8.53~8.50 (m,1H,ArH),8.29 (dt,J=7.8,1.2 Hz,1H,ArH),8.18~8.09 (m,3H,ArH),7.94 (t,J=8.1 Hz,1H,ArH),7.85~7.79 (m,1H,ArH). HRMS (AP-ESI) calcd for C14H9ClN3O2 [M+H]+ 286.0383,found 286.0377.
2-氯-4-(噻吩-3-基)喹唑啉(3d): 白色固体,产率34%. m.p. 136~138 ℃; 1H NMR (300 MHz,DMSO-d6) δ: 8.41~8.38 (m,2H,ArH),8.14~8.08 (m,1H,ArH),8.04 (m,1H,ArH),7.87~7.79 (m,2H,ArH),7.70 (dd,J=5.1,1.2 Hz,1H,ArH). HRMS (AP-ESI) calcd for C12H8Cl- N2S [M+H]+ 247.0097,found 247.0092.
2-氯-4-(噻吩-2-基)喹唑啉(3e): 白色固体,产率41%. m.p. 104~106 ℃(文献值[26] 107~108 ℃); 1H NMR (400 MHz,DMSO-d6) δ: 8.64 (d,J=8.0 Hz,1H,ArH),8.18~8.17 (m,1H,ArH),8.11~8.08 (m,2H,ArH),8.02 (d,J=8.0 Hz,1H,ArH),7.86~7.84 (m,1H,ArH),7.41 (dd,J=4.8,3.6 Hz,1H,ArH).
2-氯-4-(呋喃-2-基)喹唑啉(3f): 白色固体,产率34%. m.p. 143~145 ℃; 1H NMR (400 MHz,DMSO-d6) δ: 8.93 (d,J=8.8 Hz,1H,ArH),8.26 (s,1H),8.11 (t,J=7.2 Hz,1H,ArH),7.99 (d,J=8.0 Hz,1H,ArH),7.87 (t,J=7.2 Hz,1H,ArH),7.77 (d,J=3.6 Hz,1H,ArH),6.92 (br s,1H,ArH). HRMS (AP-ESI) calcd for C12H8ClN2O [M+H]+ 231.0325,found 231.0322.
-
-
[1]
Jatav, V.; Mishra, P.; Kashaw, S. Eur. J. Med. Chem. 2008, 43, 1945. doi: 10.1016/j.ejmech.2007.12.003
-
[2]
Goyal, M.; Sasmal, D. J. Ethnopharmacol. 2014, 151, 536. doi: 10.1016/j.jep.2013.11.011
-
[3]
Smits, R. A.; Adami, M.; Istyastono, E. P. J. Med. Chem. 2010, 53, 2390. doi: 10.1021/jm901379s
-
[4]
Jain, K. S.; Bariwal, J. B.; Kathiravan, M. K. Bioorg. Med. Chem. 2008, 16, 4759. doi: 10.1016/j.bmc.2008.02.091
-
[5]
Alagarsamy, V.; Pathak, U. S. Bioorg. Med. Chem. 2007, 15, 3457. doi: 10.1016/j.bmc.2007.03.007
-
[6]
Ismail, M. A.; Barker, S.; Abou El Ella, D. A. J. Med. Chem. 2006, 49, 1526. doi: 10.1021/jm050232e
-
[7]
Verhaeghe, P.; Azas, N.; Gasquet, M. Bioorg. Med. Chem. Lett. 2008, 18, 396. doi: 10.1016/j.bmcl.2007.10.027
-
[8]
Pandey, S. K.; Singh, A.; Singh, A. Eur. J. Med. Chem. 2009, 44, 1188. doi: 10.1016/j.ejmech.2008.05.033
-
[9]
Grover, G.; Kini, S. G. Eur. J. Med. Chem. 2006, 41, 256. doi: 10.1016/j.ejmech.2005.09.002
-
[10]
Maggio, B.; Daidone, G.; Raffa, D. Eur. J. Med. Chem. 2001, 36, 737. doi: 10.1016/S0223-5234(01)01259-4
-
[11]
Li, D.-D.; Hou, Y.-P.; Wang, W. Curr. Med. Chem. 2012, 19, 871.
-
[12]
Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Eur. J. Med. Chem. 2014, 76, 193. doi: 10.1016/j.ejmech.2014.02.005
-
[13]
Connolly, D. J.; Cusack, D.; O'sullivan, T. P. Tetrahedron. 2005, 61, 10153. doi: 10.1016/j.tet.2005.07.010
-
[14]
Hwang, D.-F.; Shiu, Y.-C.; Hwang, P.-A. J. Food Prot. 2002, 65, 1341.
-
[15]
Hwang, D.-F.; Hsieh, Y.-W.; Shiu, Y.-C. J. Food Prot. 2002, 65, 389.
-
[16]
Pao, W.; Miller, V.; Zakowski, M. Proc. Natl. Acad. Sci. 2004, 101, 13306. doi: 10.1073/pnas.0405220101
-
[17]
Raymond, E.; Faivre, S.; Armand, J. P. Drugs 2000, 60, 15. doi: 10.2165/00003495-200060001-00002
-
[18]
Wells, S. A.; Robinson, B. G.; Gagel, R. F. J. Clin. Oncol. 2012, 30, 134. doi: 10.1200/JCO.2011.35.5040
-
[19]
Backes, A.; Zech, B.; Felber, B. Expert Opin. Drug Discovery 2008, 12, 1409.
-
[20]
Backes, A.; Zech, B.; Felber, B. Expert Opin. Drug Discovery 2008, 12, 1427.
-
[21]
Mclaughlin, N. P.; Evans, P. J. Org. Chem. 2009, 75, 518.
-
[22]
Asghar, U; Witkiewicz, A. K.; Turner, N. C. Nat. Rev. Drug Discovery 2015, 14, 130. doi: 10.1038/nrd4504
-
[23]
Wang, J. H.; Wang, Q. D.; Dun, Y. Y. Chem. J. Chin. Univ. 2014, 35, 1189 (in Chinese). (王军华, 王泉德, 顿艳艳, 高等学校化学学报, 2014, 35, 1189.)
-
[24]
Sun, Z.; Wang, H.; Wen, K. J. Org. Chem. 2011, 76, 4149. doi: 10.1021/jo2003715
-
[25]
Jiang, N.; Zhai, X.; Zhao, Y. Eur. J. Med. Chem. 2012, 54, 534. doi: 10.1016/j.ejmech.2012.05.039
-
[26]
Harden, D. B. J. Org. Chem. 1988, 53(17), 4137. doi: 10.1021/jo00252a057
-
[1]
-
表 1 化合物4的结构
Table 1. The structure of the compounds 4
Compd. R1 R2 4a Ph H 4b Ph 4-CH3 4c Ph 3-NO2 4d Ph 3-Morpholino 4e Ph 4-Morpholino 4f Ph 4-SO2NH2 4g 4-CH3C6H4 4-CH3 4h 4-CH3C6H4 3-NO2 4i 4-CH3C6H4 4-Morpholino 4j 3-NO2C6H4 H 4k 3-NO2C6H4 4-CH3 4l 3-NO2C6H4 3-NO2 4m 3-NO2C6H4 4-Morpholino 4n Thiophen-3-yl H 4o Thiophen-3-yl 4-CH3 4p Thiophen-3-yl 3-NO2 4q Thiophen-3-yl 4-Morpholino 4r Thiophen-3-yl 4-CH3 4s Thiophen-3-yl 3-NO2 4t Thiophen-3-yl 4-Morpholino 4u Furan-2-yl H 4v Furan-2-yl 4-CH3 4w Furan-2-yl 3-NO2 4x Furan-2-yl 4-Morpholino 表 2 代表化合物的体外抗增殖活性
Table 2. Antiproliferative activities of representative compounds
Compd. IC50/(μmol•L-1) Hela A549 MCF-7 4u 12.76±2.36 32.95±1.17 16.21±2.00 4v 14.93±1.59 30.47±1.56 26.24±0.76 R-Roscovitine 14.01±2.62 78.70±6.03 12.72±1.25 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 0
- 文章访问数: 1102
- HTML全文浏览量: 160



 下载:
下载:

 下载:
下载:

