1 云南中医学院中药学院 昆明 650500;
2 云南中医学院第一附属医院中心实验室 昆明 650021
2016-04-24 收稿, 2016-06-01 接受
基金项目: 云南省应用基础研究项目(2014FZ087)资助
作者简介:
虎春艳, 女, 37岁, 讲师
Syntheses and Cytotoxic Activities of Chalcone Derivatives bearing N-Substituted Piperazine Moiety
1 School of Traditional Chinese Medicine, Yunnan University of Traditional Chinese Medicine, Kunming 650500;
2 Central laboratory, The NO.1 Affiliated Hospital of Yunnan University of Traditional Chinese Medicine, Kunming 650021
查尔酮类化合物广泛存在于药用植物中,普遍具有良好的生理活性,如抗菌、抗炎和抗肿瘤等活性[1-4]。因其反应位点多和毒性低的特点,近年来在新药研究方面日益受到重视[5-8]。哌嗪作为含氮杂环体系中的重要一员,具有优良的生物活性和低毒性的特点,广泛用于药物设计合成[9, 10]。我们在前期研究中发现,含苯乙酮取代哌嗪的查尔酮衍生物具有良好的体内外抗肿瘤活性[11]。在此基础上,为了进一步研究哌嗪环上不同取代基对该类化合物细胞毒活性的影响,通过引入不同的结构单元,如苯乙酮、丙酮、乙酸酯等,结合活性研究,初步阐述该类化合物的构效关系。合成路线见图式 1。
Bruker AM-400核磁共振波谱仪,TMS为内标;YANACO显微熔点仪,温度未校正;二氧化碳培养箱;AutoSpec Premier P776 型双聚焦三扇型磁质谱仪;Epoch连续波长酶标仪;RPMI-1640培养基。
称取1.38g(10mmol)4-氟苯乙酮和1.36g(10mmol)4-甲氧基苯甲醛于50mL圆底烧瓶中,加入15mL乙醇,在室温搅拌下滴加15mL 20% KOH溶液,并继续反应15h。TLC检测反应完成后,将反应物倒入50mL水中,搅拌析出固体后,真空抽滤。固体用水洗涤(20mL×3),干燥,得2.15g黄色固体,收率84%。熔点:156-158℃;1H NMR(400MHz,CDCl3)δ:8.01(d,J=8.7Hz,2H),7.81(d,J=15.3Hz,1H),7.56(d,J=8.7Hz,2H),7.31(d,J=15.3Hz,1H),7.10-7.16(m,2H),6.91(d,J=8.8Hz,2H),3.83(s,3H);13C NMR(101MHz,CDCl3)δ:188.9,166.8,165.2,152.1,146.0,135.4,130.8,130.7,130.4,122.51,115.7,115.0,115.3,111.7,51.9。
称取256mg(1mmol)上述合成的化合物1、971mg(5mmol)六水合哌嗪和276mg(2mmol)碳酸钾加入25mL圆底烧瓶中,再加入5mL二甲基甲酰胺(DMF),在120℃反应12h。TLC检测反应完成后,将反应物倒入20mL冷水中,搅拌,析出固体。真空抽滤,固体用水洗涤(10mL×3)后,干燥,得到258mg淡红色固体,收率80%。熔点:174-176℃;1H NMR(400MHz,CDCl3)δ:8.02(d,J=9.0Hz,2H),7.80(d,J=15.4Hz,1H),7.54(d,J=9.0Hz,2H),7.41(d,J=15.4Hz,1H),6.92(d,J=8.8Hz,2H),6.73(d,J=8.7Hz,2H),3.81(s,3H),3.31(t,J=4.8Hz,4H),2.54(t,J=5.2Hz,4H),1.77(s,1H);13C NMR(101MHz,CDCl3)δ:188.7,166.5,153.4,150.9,145.5,131.2,131.1,128.6,124.2,117.1,114.0,110.9,52.1,45.70,39.9;HRMS:C20H23N2O2[M+H]+ m/z,理论值324.1760,实测值324.1763。
称取97mg(0.3mmol)化合物2和0.5mL二异丙基乙胺于25mL圆底烧瓶中,加入5mL二氯甲烷(DCM)和5mL乙腈,搅拌下加入0.5mmol溴代物,室温搅拌2-5 h。TLC检测反应完成后,加入20mL DCM溶解,有机相用水洗涤(20mL×3),经无水硫酸钠干燥后浓缩,残余物以甲醇/DCM(1:99,体积比)为洗脱剂柱层析分离得到目标产物。
化合物3a:黄褐色粘稠物;1H NMR(400MHz,CDCl3)δ:8.00(d,J=8.9Hz,2H),7.81(d,J=15.3Hz,1H),7.55(d,J=9.0Hz,2H),7.43(d,J=15.3Hz,1H),6.91(d,J=8.7Hz,2H),6.74(d,J=9.0Hz,2H),3.82(s,3H),3.32(t,J=4.9Hz,4H),3.23(s,2H),2.52(t,J=5.1Hz,4H),2.14(s,3H);13C NMR(101MHz,CDCl3)δ:206.0,188.7,167.0,152.9,150.9,146.3,133.2,131.1,128.5,124.2,117.12,114.0,113.6,110.9,53.2,51.2,45.4,40.0,27.9;HRMS:C23H27N2O3 [M+H]+m/z,理论值379.2022,实测值379.2025。
表 1
Table 1
表 1(Table 1)
表 1 N-取代哌嗪查尔酮衍生物
Table 1 The structure of derivatives
|
化合物 | R | 收率/% |
|
3a | 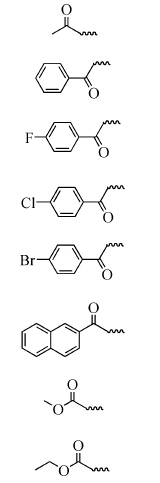 | 91 |
|
3b | 84 | |
3c | 85 | |
3d | 86 | |
3e | 86 | |
3f | 82 | |
3g | 90 | |
3h | 92 |
|
表 1 N-取代哌嗪查尔酮衍生物
Table 1 The structure of derivatives
|
化合物3b:黄色固体;熔点:179-182℃;1H NMR(400MHz,CDCl3)δ:8.01(d,J=8.4Hz,2H),7.80(d,J=15.4Hz,1H),7.51-7.56(m,3H),7.45(d,J=8.7Hz,2H),7.38(d,J=15.4Hz,1H),6.87(d,J=9.0Hz,2H),6.67(d,J=8.9Hz,2H),3.87(s,3H),3.82(s,2H),3.42(t,J=4.2Hz,4H),2.75(t,J=4.8Hz,4H);13C NMR(101MHz,CDCl3)δ:196.3,188.5,153.3,150.6,144.5,135.3,133.7,132.5,131.7,129.1,128.6,128.1,120.2,116.8,115.5,111.6,64.4,52.2,47.1。
化合物3c:淡黄色固体;熔点:180-182℃;1H NMR(400MHz,CDCl3)δ:8.01(d,J=9.0Hz,2H),7.98(d,J=8.4Hz,2H),7.77(d,J=15.4Hz,1H),7.55(d,J=8.9Hz,2H),7.37(d,J=15.3Hz,1H),7.12(t,J=8.7Hz,2H),6.91(d,J=9.0Hz,2H),6.69(d,J=9.0Hz,2H),3.87(s,3H),3.82(s,2H),3.43(t,J=4.1Hz,4H),2.74(t,J=4.8Hz,4H);13C NMR(101MHz,CDCl3)δ:195.1,188.4,164.3,161.7,153.8,151.5,145.4,133.1,131.7,131.2,130.6,130.0,127.3,122.6,115.7,114.1,113.8,113.2,111.6,63.4,53.6,51.7,46.6。
化合物3d:灰白色固体;熔点:184-186℃;1H NMR(400MHz,CDCl3)δ:8.00(d,J=8.7Hz,2H),7.97(d,J=9.0Hz,2H),7.77(d,J=15.4Hz,1H),7.54(d,J=8.7Hz,2H),7.45(d,J=8.9Hz,2H),7.40(d,J=15.4Hz,1H),6.91(d,J=9.0Hz,2H),6.68(d,J=9.0Hz,2H),3.88(s,3H),3.81(s,2H),3.40-3.44(m,4H),2.76(t,J=4.5Hz,4H);13C NMR(101MHz,CDCl3)δ:195.3,188.1,153.3,150.2,144.3,139.9,134.2,130.8,130.2,129.8,129.4,129.1,123.1,116.4,115.4,113.9,112.0,64.5,53.1,51.4,47.3。
化合物3e:淡绿色固体;熔点:179-181℃;1H NMR (400MHz,CDCl3)δ:7.99(d,J=8.7Hz,2H),7.87(d,J=9.0Hz,2H),7.79(d,J=15.3Hz,1H),7.59(d,J=9.0Hz,2H),7.55(d,J=8.7Hz,2H),7.40(d,J=15.4Hz,1H),6.90(d,J=9.0Hz,2H),6.69(d,J=8.8Hz,2H),3.86(s,3H),3.80(s,2H),3.42(t,J=4.5Hz,4H),2.72(t,J=5.0Hz,4H);13C NMR(101MHz,CDCl3)δ:196.1,187.5,153.2,151.8,144.1,134.7,131.0,130.3,130.2,129.8,129.3,128.6,123.0,116.8,113.8,111.4,64.4,53.3,51.2,47.2;HRMS:C28H28BrN2O3 [M+H]+ m/z:理论值519.1283,实测值519.1285。
化合物3f:淡黄色固体;熔点:190-192℃;1H NMR(400MHz,CDCl3)δ:8.17(s,1H),8.05(d,J=5.7Hz,1H),7.96(d,J=8.7Hz,2H),7.69(d,J=15.4Hz,1H),7.55(d,J=8.9Hz,2H),7.45(d,J=9.0Hz,2H),7.41(d,J=15.4Hz,1H),7.04-7.16(m,3H),6.91(d,J=9.0Hz,2H),6.68(d,J=8.7Hz,2H),3.90(s,3H),3.84(s,2H),3.43(s,4H),2.75-2.79(m,4H);13C NMR(101MHz,CDCl3)δ:188.4,156.2,153.1,150.4,145.9,142.3,138.8,134.0,131.7,130.8,130.5,129.9,129.6,129.2,125.6,124.1,123.5,120.6,116.7,113.9,112.2,111.7,110.6,64.5,53.1,51.9,51.1,47.7。
化合物3g:黄色固体;熔点:170-172℃;1H NMR(400MHz,CDCl3)δ:8.01(d,J=8.7Hz,2H),7.80(d,J=15.3Hz,1H),7.58(d,J=8.9Hz,2H),7.41(d,J=15.4Hz,1H),6.91(d,J=8.7Hz,2H),6.69(d,J=9.0Hz,2H),4.11(s,3H),3.83(s,3H),3.40-3.46(m,4H),3.28(s,2H),2.74(t,J=4.5Hz,4H);13C NMR(101MHz,CDCl3)δ:188.2,170.8,153.1,151.6,141.5,133.6,130.2,125.7,123.2,116.9,114.4,112.2,111.7,60.9,58.7,53.3,52.0,47.5。
化合物3h:淡褐色固体;熔点:171-173℃;1H NMR(400MHz,CDCl3)δ:8.00(d,J=8.4Hz,2H),7.79(d,J=15.4Hz,1H),7.56(d,J=9.0Hz,2H),7.39(d,J=15.3Hz,1H),6.90(d,J=9.0Hz,2H),6.68(d,J=8.7Hz,2H),4.20(q,J=7.0Hz,2H),3.80(s,3H),3.43(t,J=4.5Hz,4H),3.27(s,2H),2.72(t,J=4.3Hz,4H),1.30(t,J=7.2Hz,3H);13C NMR(101MHz,CDCl3)δ:188.7,171.1,152.6,151.7,144.3,131.1,130.7,129.3,123.2,120.6,116.9,113.8,113.4,111.2,61.5,58.4,53.1,51.7,47.3,14.9;HRMS:C24H29N2O4[M+H]+ m/z:理论值409.2127,实测值409.2130。
以顺铂(DDP)为阳性对照,以A549和SGC7901为待测细胞株,采用四甲基偶氮唑盐比色法(MTT法)测定目标化合物的体外抗肿瘤活性。取对数生长期的肿瘤细胞(5×104个/mL)接种于96孔板中,培养过夜后加入化合物,设置6个浓度梯度,每个浓度3个复孔。作用48h后,每孔加入20μL 5mg/mL的MTT,继续培养4h,于3000r/min离心10min,吸弃培养液,加入150μL DMSO,于震荡器震荡10min,计算IC50,结果见表 2。
表 2
Table 2
表 2(Table 2)

表 2 化合物的体外细胞毒活性
Table 2 IC50 value of compounds against human tumor cell lines
|
Compound |
Cell lines (IC50,μmol/L) |
|
A549 | SGC7901 |
|
3a | 0.28 | 2.53 | |
3b | 23.41 | >40 | |
3c | 14.05 | 20.16 | |
3d | 9.03 | 11.28 | |
3e | 18.74 | 28.34 | |
3f | >40 | 37.45 | |
3g | >40 | >40 | |
3h | >40 | >40 | |
DDP | 11.54 | 12.44 |
|
表 2 化合物的体外细胞毒活性
Table 2 IC50 value of compounds against human tumor cell lines
|
在前期研究中,哌嗪环NH与α-溴代酮进行取代反应时采用K2CO3为碱。虽然在该条件下反应速度较快,但副产物较多,不利于产物的纯化。在本研究中,我们对反应条件进行了优化。以合成化合物3a为例,考察了不同碱和溶剂对反应的影响,结果发现当以二异丙基乙胺为碱,CH3CN/DCM=1:1(体积比)为溶剂时,反应效果较好,见表 3。
表 3
Table 3
表 3(Table 3)

表 3 碱对化合物3a的影响
Table 3 Effect of bases on the yield
|
Entry | Base | Solvent | Yield/% |
|
1 | Et3N | DCM | 37 | |
2 | Et3N | CH3CN/DCM=1:1 | 56 | |
3 | DIPEA | DCM | 76 | |
4 | DIPEA | CH3CN/DCM=1:1 | 91 | |
5 | K2CO3 | DCM | 81 | |
6 | K2CO3 | CH3CN/DCM=1:1 | 74 | |
7 | Cs2CO3 | DCM | 68 | |
8 | Cs2CO3 | CH3CN/DCM=1:1 | 62 |
|
表 3 碱对化合物3a的影响
Table 3 Effect of bases on the yield
|
从表 1可以看出,该类化合物对肿瘤细胞株A549和SGC7901均表现出较好的细胞毒活性。从结构上来看,哌嗪环上含丙酮和苯乙酮片段的衍生物对肿瘤细胞的抑制活性要优于含酯基的,并且当苯环上有卤素取代时活性会增强。在所有化合物中,化合物3a对肿瘤细胞的细胞毒活性最强,对A549和SGC7901的IC50值分别为0.28μmol/L和2.53μmol/L。
本文通过3步反应合成得到了8个新的查尔酮哌嗪衍生物,体外细胞毒活性测试表明,哌嗪环上取代有含酮片段的化合物对肿瘤细胞株A549和SGC7901均表现出良好的抑制活性。
 2016,
Vol. 79
2016,
Vol. 79 Issue (11): 1089-1092
Issue (11): 1089-1092 2016,
Vol. 79
2016,
Vol. 79 Issue (11): 1089-1092
Issue (11): 1089-1092